PHILADELPHIA - A study on amyloid plaque accumulation, white-matter hyperintensity, and cortical thickness has found that a patient's cerebrovascular characteristics should be considered for their potential effect on the progression of Alzheimer's disease. The findings were presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
While cerebrovascular disease currently is not considered a primary cause of Alzheimer's disease, the study revealed that increased amyloid pathology and white-matter hyperintensity volume correlated with lower cortical thickness. All three of these factors are associated with mild cognitive impairment and Alzheimer's disease.
"There should be an explicit consideration of occurrence of cerebrovascular disease on the pathogenesis of Alzheimer's disease, especially since in the real world, dementia is due to mixed pathologies," said study author Patrick Lao, a postdoctoral researcher at Columbia University Medical Center.
Risk factors
The reason for studying Alzheimer's disease and cerebrovascular disease together is that "they are both strongly correlated with age and they share the same vascular risk factors," Lao told attendees at SNMMI 2018. "They also can lead to similar patterns of neurodegeneration and cognitive dysfunction, making them hard to distinguish clinically."
While previous research has typically investigated these two conditions separately, Lao was interested in connections such as how small-vessel cerebrovascular disease can affect Alzheimer's pathophysiology through impaired perivascular clearance, or how Alzheimer's pathophysiology can affect small-vessel cerebrovascular disease through direct cytotoxic effects.
"It also could be that small-vessel cerebrovascular disease has an interaction where it lowers the threshold of clinical expression of dementia at a given burden of Alzheimer's pathology," Lao said.
Lao collected data from 608 subjects in the Alzheimer's Disease Neuroimaging Initiative (ADNI). That total included 222 control subjects, 291 people with mild cognitive impairment, and 93 Alzheimer's patients. He also noted that this research was framed as a pure Alzheimer's disease study, so participants only had a low to moderate vascular risk.
Through their participation in the ADNI, subjects underwent longitudinal PET imaging with the radiopharmaceutical florbetapir (Amyvid/AV45, Eli Lilly) to quantify amyloid plaque in the brain, as well as T1- and T2-weighted MRI scans to measure white-matter hyperintensity and cortical thickness, respectively. White-matter hyperintensity volume can be an indication of cerebrovascular abnormalities, while cortical thickness measurements were averaged across nine brain regions that are known to show atrophy related to Alzheimer's disease.
"The proposed mechanism that links hyperintensities to small-vessel cerebrovascular disease is some form of vessel damage, which leads to chronic hypoperfusion and to demyelination," Lao added.
In addition, standardized uptake value ratios (SUVRs) for florbetapir were calculated as the ratio of cortical regions to a composite white-matter reference region.
Finally, Lao used a series of linear models to test for the following:
- Differences in florbetapir SUVR, white-matter hyperintensity volume, and cortical thickness between diagnostic groups
- The relationship among the three image-derived biomarker measures: amyloid, cerebrovascularity, and neurodegeneration
- Differences in rates of change over time in the biomarkers across diagnostic groups
- The relationship between longitudinal rate of change in cortical thickness, an indication of neurodegeneration, and baseline amyloid or white-matter hyperintensity volume
Biomarker correlations
In reviewing the results, Lao found a statistically significant increase in amyloid burden in patients with mild cognitive impairment and Alzheimer's, compared with the control subjects. In addition, there was a statistically significant slowdown in the rate of amyloid accumulation between the normal controls and those with mild cognitive impairment, compared with the Alzheimer's group.
"High amyloid burden increases across the clinical diagnostic groups, but there are 15% of clinically diagnosed Alzheimer's subjects who do not have high amyloid," Lao said. "That might indicate what is happening in the slowing of amyloid burden in the Alzheimer's group."
Hyperintensity volumes also increased from normal controls to mild cognitively impaired and Alzheimer's patients, but not to a significant degree.
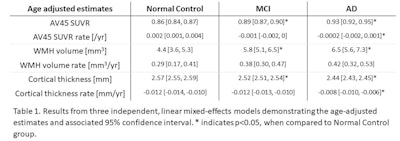 MCI = mild cognitive impairment; AD = Alzheimer's disease; WMH = white-matter hyperintensities. Image courtesy of Patrick Lao.
MCI = mild cognitive impairment; AD = Alzheimer's disease; WMH = white-matter hyperintensities. Image courtesy of Patrick Lao.As expected, cortical thickness decreased across the three diagnostic groups, with a statistically significant difference between the control group and subjects with mild cognitive impairment and Alzheimer's. The rate of cortical thickness decline was most severe and statistically significant among Alzheimer's patients.
Lao also used a series of linear and growth curve models to test for associations between biomarkers. The approach allowed for analyses of one biomarker at baseline compared with the rate of change in another biomarker. In one model that included all three imaging biomarkers, increased florbetapir SUVR and white-matter hyperintensity volume were both significantly associated with decreased cortical thickness. In addition, the longitudinal cortical thinning rate was significantly associated with higher baseline white-matter hyperintensity but not florbetapir SUVR.
Looking ahead, Lao said that future work in this area should include "studying the same associations but in understudied racial and ethnic minority populations with greater vascular risk."