Major changes are taking place in the intricate network that supplies healthcare providers around the world with molybdenum-99 (Mo-99), a key radioisotope for nuclear medicine studies. The question is, can the nuclear medicine community avoid another devastating shutdown like the one that occurred in 2009?
Back then, providers were left scrambling after a perfect storm left sites without supplies of Mo-99, which cannot be stockpiled due to its extremely short half-life. In the years since the 2009 crisis, nuclear reactor operators, nuclear medicine pharmacies and practitioners, radioisotope generator manufacturers, medical societies, and other stakeholders have banded together to ensure that adequate supplies of Mo-99 and its technetium-99m (Tc-99m) byproduct are consistently available.
"This whole occurrence of 2009 really presented a wake-up call to the industry at large, but I think we have embraced it," said Sally Schwarz, co-director of the cyclotron facility at the Washington University School of Medicine in St. Louis. "This has been an extensive and time-consuming process to move this change forward."
 Sally Schwarz from Washington University School of Medicine in St. Louis.
Sally Schwarz from Washington University School of Medicine in St. Louis.One of the more significant steps to Mo-99 stability was the creation in 2009 of a joint effort between the Organization for Economic Cooperation and Development (OECD) and the Nuclear Energy Agency (NEA). The goal is to coordinate Mo-99 production and develop ways to ensure that supply meets worldwide demand; the organizations' members cover the gamut of nuclear medicine enthusiasts and meet every six months in Paris.
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine (EANM) are also on hand to advocate for physicians who use radioisotopes in the treatment of their patients.
One of the tenets of the OECD-NEA collaborative is to develop an outage reserve capacity. That means Mo-99 suppliers must have permanent arrangements in place to acquire additional capacity to cover shortfalls when reactors go offline for scheduled or unplanned maintenance.
"On the recommendations of the NEA and OECD, there is a 35% contingency supply," said Cathy Cutler, PhD, director of the Medical Isotope Research and Production (MIRP) program at the Brookhaven National Laboratory. "If someone goes down, you can call on this 35%. They are basically asking radiators to produce this additional amount."
The outage reserve capacity is maintained by Mo-99 suppliers paying for extra ports and time on a nuclear reactor. If a supply issue occurs, they then have a position in line to acquire additional Mo-99 to cover the shortfall.
Supply and demand
The demand for Mo-99 is expected to grow modestly over the next four years, peaking at approximately 11,500 Ci per week. Interestingly, the anticipated upswing follows a reduced call for Mo-99 in 2017. Currently, the OECD estimates that worldwide demand for Mo-99 is at 9,000 six-day Ci per week. Mature markets account for approximately 84% of the demand, while emerging markets take the remaining 16%. The growth rate in mature markets is expected to remain stable at 0.5% through 2021.
Whatever demands are to come will be handled by fewer Mo-99 suppliers, however. On March 31, the National Research Universal (NRU) nuclear reactor in Chalk River, Ontario, Canada, went offline for the final time after serving North America for decades. Even before its permanent closure, the NRU reactor manufactured little if any Mo-99 since October 2016. In addition, the Osiris reactor in France shut down at the end of 2015. Their departure leaves four Mo-99 manufacturers who now must cover the loss.
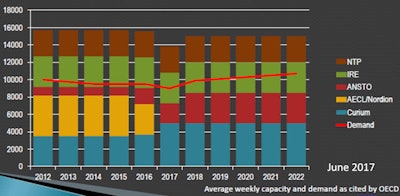 Mo-99 production capacity at major producers (six-day Ci per week). Image courtesy of Sally Schwarz.
Mo-99 production capacity at major producers (six-day Ci per week). Image courtesy of Sally Schwarz.Australian Nuclear Science and Technology Organization (ANSTO)
ANSTO's Open Pool Australian Lightwater (OPAL) nuclear reactor currently produces some 2,300 Ci per week. With the help of a $168 million ($128 million U.S.) investment from the Australian government, ANSTO is in the process of completing work on its state-of-the-art ANSTO Nuclear Medicine (ANM) Mo-99 production facility. ANM this month received its license to operate and begin testing its Mo-99 production process in May.
"It involves introducing the irradiated low-enriched uranium target plates from the OPAL reactor to the hot cell, extracting and purifying Mo-99 from them, and going through various medicine manufacturing sequences, such as quality control checks," Jane Senior, general manager for ANM, wrote in an email to AuntMinnie.com.
ANM will take over the primary Mo-99 manufacturing duties from the OPAL reactor, with production increasing to approximately 3,500 six-day Ci per week at full capacity, which is scheduled to occur before the end of this year. That amount would allow ANSTO to serve Australia's needs and potentially accommodate approximately 25% of global demand.
ANM's ability to perform three Mo-99 runs in one day, rather than one run per day at OPAL, is also expected to provide additional supplies during peak weekend periods.
Curium
Nuclear medicine firm Curium was created in April 2017, three months after IBA Molecular's acquisition of Mallinckrodt Nuclear Medicine. The entity's roots date back to 1996 when Dutch radiopharmaceutical producer Nuclear Research and Consultancy Group (NRG) began Mo-99 production at its High Flux Reactor (HFR) in Petten.
Today, Curium takes its Mo-99 supply from the HFR; the Belgian Reactor 2 (BR2) in Mol, Belgium; and the Maria reactor in Warsaw, Poland. Last year, Curium increased its take from four to five runs per week, which brought its capacity to approximately 4,500 Ci per week. If necessary, Curium could increase its output to six productions per week.
In addition, Curium has outage reserve capacity arrangements with the three reactor operators, which could add approximately 100 weeks of supply during a Mo-99 shortage. And if one of those reactors goes offline, one of the other two facilities can make up the difference.
NTP Radioisotopes
NTP Radioisotopes in South Africa has its own dedicated nuclear reactor that manufactures a number of targets, including Mo-99. The Safari-1 reactor processing facility completed its conversion to low-enriched uranium (LEU) last September. The reactor's capacity for Mo-99 is in the range of 1,400 Ci per week.
"We want to be in nuclear medicine and the technetium-99m supply business for many years to come," commented Piet Louw, executive manager for NTP Radioisotopes, during a June 2017 SNMMI forum on Mo-99. "We believe that 20 years from now there will still be a big demand for technetium, and we want to be there to service this demand. We are committed to it and we are making the necessary investments."
Institute for Radioelements (IRE)
While it is capable of greater capacity, IRE's license allows for three days of Mo-99 production or 3,500 Ci per week using an LEU process. That output represents approximately 25% of the weekly global demand. IRE also has contracts to take Mo-99 supply from the HFR in the Netherlands, the BR2 in Belgium, and the LVR-15 reactor in the Czech Republic.
"The problem we faced in 2009 and 2010 was not a capacity problem, because the capacity was there," Jean-Michel Vanderhofstadt, CEO of IRE, recalled at the SNMMI event. "The problem was reliability. Reliability is the key in this business."
Mo-99 alternatives
Demand for Mo-99 has held fairly steady since the shortage in 2009. One reason for the decline last year may be that nuclear medicine practitioners are wary of history repeating itself and believe they should have other radioisotope options.
"I think part of that [decrease in 2017] is because people have been looking for other isotopes they can use, so they are not so strongly dependent on Mo-99 because of the shortages," Cutler said.
Among its tasks, Brookhaven's MIRP program distributes certain commercially unavailable radioisotopes to the nuclear medicine community and develops new radioisotopes for nuclear medicine research. The facility has an accelerator to produce radionuclides and isotopes for imaging.
"One of the prominent uses for Mo-99 is for imaging the heart. Another agent that can be used to do that is strontium-rubidium, which we can make on the accelerator," Cutler said. "The demand [for strontium-rubidium] has been higher than what is produced. The use of rubidium has been increasing because of the [Mo-99] supply problems."
At Washington University in St. Louis, there are four clinical cyclotrons that manufacture F-18 FDG, nitrogen-13 ammonia, technetium-99m sestamibi, and carbon-11 choline, among others. The radiotracers are primarily used onsite and not distributed unless the facility receives a call from a nearby imaging center.
"Although nuclear medicine is not growing rapidly, it still is a very important diagnostic tool and we have a very broad base in the U.S.," Schwarz said. "We certainly need to be thinking about how we can produce Mo-99 and have technetium-99m."