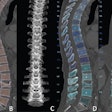
GE Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Gemstone spectral imaging (GSI) Xtream technology on its Revolution CT scanners.
GSI Xtream features wide collimation and a 50-cm field-of-view for volume spectral CT for small lesion detection, tissue characterization, and metal artifact reduction, the company said.
GSI Xtream editions of the Revolution CT scanner are installed at Duke University Medical Center in Durham, NC; Robarts Research Institute in London, Ontario, Canada; and the First Affiliated Hospital of Dalian Medical University in China.