Minnies winners, page 3
Scientific Paper of the Year
Winner: Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. Rafferty EA et al, Journal of the American Medical Association, April 26, 2016. To learn more about this paper, click here.
By selecting this study as Scientific Paper of the Year, the Minnies expert panel acknowledged the rising role of digital breast tomosynthesis (DBT) for mammography screening.

For the award-winning paper, published April 26, 2016, in the Journal of the American Medical Association, Dr. Elizabeth Rafferty of L&M Radiology in West Acton, MA, led a team of researchers who wanted to assess the performance of tomosynthesis in a screening environment. In particular, they wanted to build on a 2014 study that found that DBT increased the detection of invasive breast cancer and reduced false positives compared to mammography alone.
For the new study, Rafferty et al examined the impact of tomosynthesis in detecting cancer in women across four varying levels of breast tissue density. As is well-known, conventional mammography has difficulty identifying cancer in women with dense breast tissue.
The researchers analyzed data from over 450,000 mammography exams originally acquired for the 2014 study. They found that tomosynthesis not only improved breast cancer detection by 48% in women with the two categories of tissue considered to be dense, but it also reduced recall rates and the number of false positives detected.
Rafferty and colleagues also analyzed data from each of the four categories to parse out differences in cancer detection rates, recall rates, and false positives, with the goal of determining where tomo might fit in the armamentarium of supplemental modalities available for use as an adjunct to mammography. They found differences in cancer detection metrics that could form the basis for future studies.
Rafferty believes that the study answers the question of whether tomosynthesis is an appropriate screening choice for all women -- she believes the answer is an unequivocal "yes."
"Tomosynthesis dramatically improves the performance of breast cancer screening from women with nondense as well as dense breasts," she told AuntMinnie.com. "The biggest bang for your buck came for women with dense breast tissue."
As the use of tomosynthesis becomes more common in developed countries, it will probably shed its role as a niche technology best used for difficult cases or supplemental imaging, and instead become the gold standard for breast imaging across a variety of patient groups.
"This paper shows that tomosynthesis is the right mammographic screening method for everyone," Rafferty concluded. "It improves all the pertinent outcomes, the true positives and the false positives."
Runner-up: Cancer risks in U.S. radiologic technologists working with fluoroscopically guided interventional procedures, 1994-2008. Rajaraman P et al, American Journal of Roentgenology, May 2016. To learn more about this paper, click here.
Best New Radiology Device
Winner: OnSight 3D extremity imaging CT scanner, Carestream Health
The winning entry in the Best New Radiology Device category this year started out as a research project at Carestream Health that eventually evolved into a commercial product.
Carestream is best known for its image management and advanced visualization software, as well as its line of digital x-ray systems. But several years ago the firm began investigating whether it could take conebeam CT (CBCT) technology being sold by its Carestream Dental unit and adapt it for clinical imaging, according to Helen Titus, marketing director for digital capture solutions at the firm.
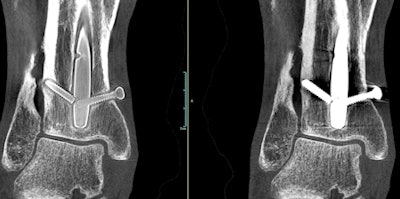 A weight-bearing extremity scan acquired with OnSight 3D. Screw threads are more visible in the image at left after metal artifact correction is applied. Image courtesy of Carestream Health.
A weight-bearing extremity scan acquired with OnSight 3D. Screw threads are more visible in the image at left after metal artifact correction is applied. Image courtesy of Carestream Health."Our research scientists thought we could leverage that technology for 3D extremity imaging," Titus told AuntMinnie.com. "It was one of those research projects that Carestream allows us to do that may or may not be commercialized."
As development proceeded, Carestream realized it could have a winner on its hands: a compact CBCT scanner capable of being sited in a physician's office that could perform weight-bearing studies not possible on existing whole-body CT scanners. Carestream received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its OnSight 3D scanner in August 2016, and shipments are scheduled to begin by the end of this year.
OnSight 3D is designed to bring advanced imaging to the orthopedic physician office segment, which up to now has mostly made do with x-ray. It includes image processing algorithms optimized for orthopedic use, such as fast 3D rendering and metal artifact reduction.
The orthopedic office segment represents a growth opportunity for Carestream, but the firm will be able to leverage the field sales and service operations that have been selling x-ray systems into these accounts. The company also sees market potential in hospitals that might want to supplement its existing whole-body CT systems with a dedicated orthopedic CT scanner.
OnSight 3D is currently in operation at two clinical sites in the U.S. and one in Finland, with another two scheduled for installation shortly. These units are generating feedback that the company will use to fine-tune its approach in preparation for official shipments beginning by the end of the year, Titus said.
Runner-up: Affirm prone breast biopsy system, Hologic
Best New Radiology Software
Winner: 4D Flow, Arterys
This year's winner comes from newcomer Arterys, who also won the 2016 Minnie for Best New Radiology Vendor (see below). 4D Flow uses cloud-based image processing technology to provide visualization and quantification of blood flow on cardiac MRI studies.
With 4D Flow, cardiac MRI exam times can be sharply reduced to just 10 minutes, according to the company. After the software is connected to a standard MRI scanner, a simple MRI exam of the chest can be automatically uploaded to the firm's cloud server without transmitting patient-protected health information outside of the hospital.
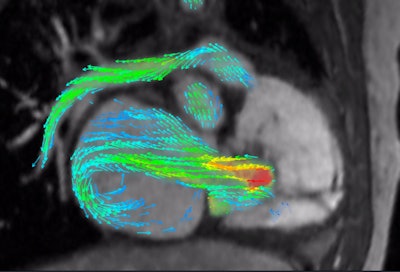 4D Flow depicts severe regurgitation on right-ventricular three-chamber view. Image courtesy of Arterys.
4D Flow depicts severe regurgitation on right-ventricular three-chamber view. Image courtesy of Arterys."That can replace a whole slew of conventional [MR] sequences that could take an hour to acquire," said John Axerio-Cilies, Arterys' co-founder and chief technology officer.
Physicians can then use a web browser to view the quantitative clinical analysis of blood flow and interact with the image data, he said.
"It can really augment clinical ultrasound when you're looking at structural heart defects or disease [for example]," he said.
Arterys first introduced 4D Flow in collaboration with GE Healthcare at RSNA 2015, and it is currently teaming up with GE on a prelaunch pilot introduction at 15 to 20 sites around the world, according to the firm. 4D Flow will initially be incorporated into GE's ViosWorks cardiac software package.
Arterys will formally launch 4D Flow next month at RSNA 2016. Deep-learning capabilities will be added later to 4D Flow once the company receives U.S. Food and Drug Administration (FDA) clearance for that technology, said Carla Leibowitz, Arterys' head of strategy and marketing.
Runners-up: ICE-T inpatient cost evaluation tool, Harvey L. Neiman Health Policy Institute; PACS Redefined, Lexmark International
(Two candidates tied for second place in this category.)
Best New Radiology Vendor
Winner: Arterys
Arterys was founded in 2011 with the vision of commercializing research software that had been developed at Stanford University for visualizing and measuring blood flow on cardiac MR images. The resulting software, called 4D Flow, is also the winner of the 2016 Minnie for Best New Radiology Software (see above).
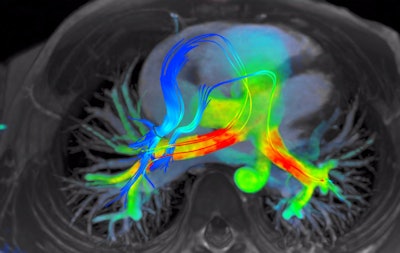 4D Flow software for cardiac MRI shows streamlines of shunted pulmonary venous flow back out to each pulmonary artery. Image courtesy of Arterys.
4D Flow software for cardiac MRI shows streamlines of shunted pulmonary venous flow back out to each pulmonary artery. Image courtesy of Arterys.Accomplishing the company's vision of making 4D Flow a clinical product required the use of cloud infrastructure, as it's not feasible to deliver the required computing power for the software to every local institution, said John Axerio-Cilies, Arterys' co-founder and chief technology officer. The company's platform -- called Arterys Cloud -- is the engine that supports widespread adoption of 4D Flow.
"We also have a much larger vision than that and plan to develop other applications on the platform," Axerio-Cilies said.
The company is preparing for the global launch of 4D Flow in partnership with GE Healthcare. The two firms inked a nonexclusive partnership late last year and showcased the Arterys software as part of GE's ViosWorks cardiac software package at RSNA 2015. 4D Flow is set to be formally launched next month at RSNA 2016, with deep-learning and artificial intelligence capabilities set to be added later upon receipt of U.S. Food and Drug Administration approval.
In addition to adding more cardiac MR functionality to 4D Flow, Arterys plans to develop other applications on its Arterys Cloud platform, including investigating opportunities in oncology and neurology. Other technology currently being developed for radiology includes longitudinal tracking of anatomy with the ability to detect changes, multiparametric image processing, and -- in a long-term project -- building predictive analytics based on phenotypes of different conditions, Arterys said.
"The idea is to give clinicians tools to help them do their jobs much faster and in a much more informed way," said Carla Leibowitz, Arterys' head of strategy and marketing.
Runner-up: 4Dx
Best Radiology Mobile App
Winner: CTisus Critical Diagnostic Measurements in CT (iOS), Dr. Elliot Fishman
The third time was the charm for Dr. Elliot Fishman of Johns Hopkins University. After being named a finalist for Best Radiology Mobile App in 2014 and 2015 for other apps in the CTisus family, Fishman finally broke through in 2016 to win with CTisus Critical Diagnostic Measurements in CT.
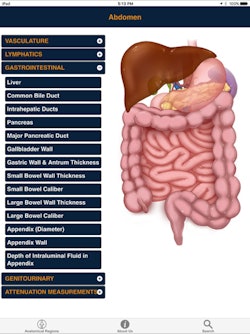 CTisus Critical Diagnostic Measurements in CT
CTisus Critical Diagnostic Measurements in CTThis year's Best Radiology Mobile App is based on the concept that in order to interpret CT exams you need to know both normal anatomy and the alterations in size and attenuation that indicate pathology. The app provides normal measurements for a range of anatomic structures, as well as critical size and Hounsfield unit thresholds for distinguishing benign lesions from potentially malignant or life-threatening pathology, according to Fishman. What's more, the app also helps users expand their knowledge base by facilitating access to the literature used to derive this information.
Fishman credited the idea for the app to Dr. Pamela Johnson, residency program director at Johns Hopkins and herself a 2016 Minnies winner for Most Effective Radiology Educator. As is the case with all CTisus apps, CTisus Critical Diagnostic Measurements in CT is free to use.
"I feel like it's our contribution to radiology," Fishman said. "We try to develop apps that people really find helpful."
More CTisus apps are also on the way. At next month's RSNA 2016 meeting in Chicago, Fishman and his team -- which includes Rachel Thomas, Sara Raminpour, Hannah Ahn, and Johnson -- will present a new app that focuses on pancreatic tumors.
"It's a team effort," Fishman said. "You have to have a good team or this doesn't happen."
An app targeted at adrenal tumors is also expected to be ready by the end of the year, Fishman said.
Notably, this is Fishman's fifth Minnie and third different Minnie category. He previously won Minnies for Most Effective Radiology Educator (2014, 2007, and 2001) and Most Influential Radiology Researcher (2004).
Runner-up: Kanal's MR Safety Implant Risk Assessment (iOS), Dr. Emanuel Kanal
Best Radiology Image
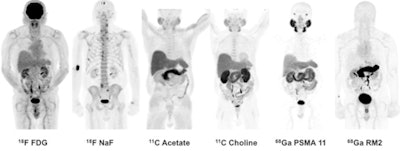
Winner: PET image series with gallium-68 PSMA-11 and gallium-68 RM2 radiopharmaceuticals
The winner of the Best Radiology Image competition in this year's Minnies awards offers a potential solution to a thorny clinical problem: determining whether and where cancer is recurring in patients with rising levels of prostate-specific antigen (PSA).
Existing imaging modalities such as CT, ultrasound, and MRI are already used to determine prostate cancer recurrence, but they have limited sensitivity and specificity. Targeted PET radiopharmaceuticals could take advantage of the modality's ability to home in on the site of disease, but some radiotracers, such as choline-11, have disadvantages of their own, including short half-lives.
The award-winning image originally ran as part of a paper published in the April 2016 edition of the Journal of Nuclear Medicine. In the study, researchers from Stanford University demonstrated their experiences with two new PET radiotracers, gallium-68 PSMA-11 and gallium-68 RM2, in a small population of patients. The study builds on earlier work with the radiotracers, namely at Heidelberg University for gallium-68 PSMA-11 and at radiopharmaceutical developer Piramal Imaging for gallium-68 RM2.
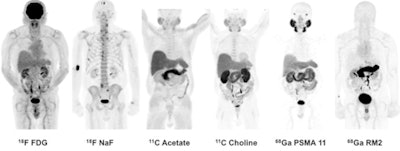
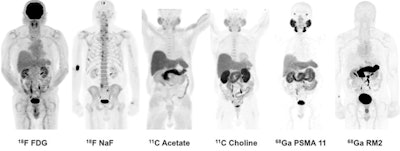
Images are of 83-year-old man with biochemically recurrent prostate cancer based on PSA levels. Images at far right are positive gallium-68 PSMA-11 and gallium-68 RM2 PET scans, both showing retroperitoneal lymph nodes while other studies are negative.
The Stanford group examined both radiotracers and compared them with other imaging methods in seven men with biochemically recurrent prostate cancer, according to Dr. Andrei Iagaru, senior author of the study and chief of the division of nuclear medicine and molecular imaging at Stanford. Because the two agents target different biological processes, the researchers wanted to see how they behaved in patients to determine the best ways to use them clinically.
The award-winning image was acquired from an 83-year-old man with a T2b nodule and PSA level of 5.4 who was treated with intensity-modulated pelvic radiotherapy and androgen blockade. After treatment, the patient's PSA level fell to a nadir of 0.11, but afterward it rose to 1.83, classifying him as being in recurrence.
Follow-up imaging was negative for cancer, however. The patient was followed for 40 months and his PSA rose from 2.72 to 18.7, at which point he received gallium-68 PSMA-11 and gallium-68 RM2 scans. These identified areas of high uptake in the retroperitoneal lymph nodes, as well as other locations.
In their discussion of the results, the authors pointed out that gallium-68 PSMA-11 and gallium-68 RM2 had different distribution patterns in the body, a finding that could guide their future use. Some patients might benefit from having both scans performed, however. What's more, the agents could be labeled with lutetium-177 for therapeutic uses.
Neither of the gallium-68 agents have been approved by the U.S. Food and Drug Administration (FDA), Iagaru said, but various groups are working on this, so the radiotracers can be used clinically.
"We need to get the community to work together to get the FDA to approve them," Iagaru said. "There is a need in the U.S. to have such imaging agents."
Runner-up: 3D volume-rendering CT image shows numerous large packets of narcotics concealed in a body packer (smuggler)



















