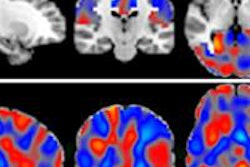
MR-guided focused ultrasound (MRgFUS) developer InSightec has received approval from the U.S. Food and Drug Administration (FDA) for its ExAblate Neuro device to treat essential tremor.
ExAblate Neuro uses high-intensity focused ultrasound (HIFU) to ablate target tissue, with MRI used during the procedure to visualize the patient's anatomy, plan treatment, and monitor outcomes. ExAblate Neuro is the first focused ultrasound device to receive FDA approval to treat essential tremor in patients who have not responded to medication, according to InSightec.
The FDA's action is based in part on clinical studies that found approximately 50% improvement in tremors and motor function three months after treatment, compared with patients' baseline scores and control subjects who showed no improvement.
Twelve months after the procedure, the treatment group retained a 40% improvement in these scores over baseline, according to the company.