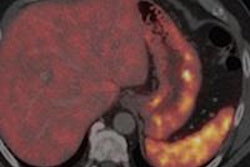
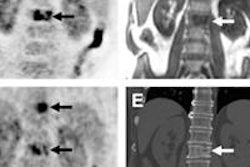
Advanced Accelerator Applications (AAA) has received orphan drug designation status for gallium-68 DOTATATE.
Both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) granted the designation for use of the radiopharmaceutical as a diagnostic agent for managing gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
AAA said the designation should help accelerate development of the agent for the benefit of GEP-NET patients in the U.S. and Europe.
Gallium-68 DOTATATE will be prepared using AAA's patented kit, which is reconstituted in hospital radiopharmacies without the use of a radiochemistry module. The product would be available to all hospitals, even facilities that do not have a fully equipped GMP production radiopharmacy unit.