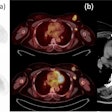
Siemens Healthcare said the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the company's RT Pro edition software for enhancing radiation oncology workflow.
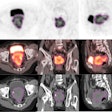
The package delivers better image quality for radiation oncology patients, including large patients, those with metal implants, and those who experience tumor motion during treatment, Siemens said.
New features in the RT Pro edition include a metal artifact reduction application. The technology improves CT data for treatment planning by reducing beam-hardening artifacts caused by metal implants.
In addition, respiratory gating features t-minIP and t-MIP enable visualization of tumors in a single image, which is useful for analyzing tumor motion and contours, the company said.
Finally, Siemens' HD FoV Pro algorithm uses intelligent contour and attenuation estimation to improve visibility outside the scanner field-of-view through an extended 80-cm field-of-view, increasing image accuracy for treatment planning.