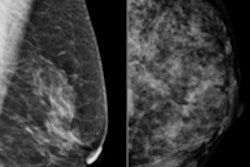
Women's imaging vendor Hologic said it has received approval from the U.S. Food and Drug Administration (FDA) for its C-View 2D imaging software, to be used with its Selenia Dimensions 3D breast tomosynthesis system.
C-View 2D images may now be used in place of the conventional 2D exposure previously required as part of a Hologic breast tomosynthesis screening exam, according to the firm.
The software generates 2D images from the 3D data acquired during a breast tomosynthesis exam, eliminating the need for additional 2D exposures and, therefore, reducing radiation dose and exam time, Hologic said.
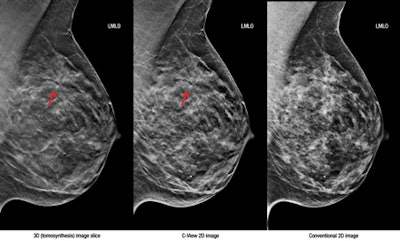
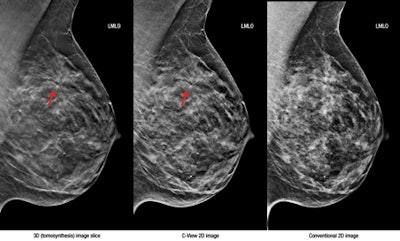 Left, 3D tomosynthesis image slice; middle, C-View 2D image; right, conventional 2D image. Images courtesy of Hologic.
Left, 3D tomosynthesis image slice; middle, C-View 2D image; right, conventional 2D image. Images courtesy of Hologic."Large-scale clinical studies have shown that screening with Hologic's 3D mammography technology allows radiologists to visualize the breast in greater detail than with 2D mammography alone, which results in earlier detection of cancers while at the same time reducing the false positives associated with conventional 2D mammography that cause unnecessary anxiety and cost," the company said in a statement.