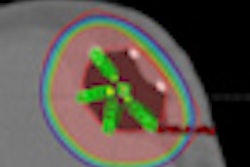
The treatment and outcomes of more than 100 breast cancer patients who received radiotherapy treatments delivered by a multichannel single-entry brachytherapy device have been so positive that radiation oncologists are suggesting that its use be expanded.
Accelerated partial-breast irradiation (APBI) treatments significantly shorten radiotherapy treatment time for low-risk women with early-stage breast cancer. But current eligibility requirements have excluded many patients who would elect this treatment.
Radiation oncologists from Arizona Oncology Services in Phoenix and the Moores Cancer Center of the University of California, San Diego (UCSD) in La Jolla reported data on dosimetry, toxicity, and cancer recurrence of 102 patients who received accelerated APBI using a strut-adjusted volume implant (SAVI) brachytherapy applicator between November 2006 through March 2009. Their findings were published online in the International Journal of Radiation Oncology, Biology, Physics (June 21, 2010).
When the first single-entry brachytherapy device (MammoSite, Hologic, Bedford, MA) first received U.S. Food and Drug Administration 501(k) clearance as a single-entry, single radiation source device in May 2002, its use was restricted from most women who either had small breasts or a tumor located close to the chest wall and lung, or the surface of the skin.
Some women with these exclusion characteristics became eligible with the introduction of multicatheter single-entry brachytherapy devices (SAVI, Cianna Medical, Aliso Viejo, CA; Contura, SenoRx, Irvine, CA). These had the ability to be expanded to conform to the site of the tumor cavity and deliver radiation sources at different strengths using multiple catheters.
Co-authors Robert Kuske, MD, of Arizona Breast Cancer Specialists in Phoenix and Catheryn Yashar MD, chief of breast and gynecological radiation services at UCSD, were the first two radiation oncologists to use the SAVI applicator. After following their patients for a median of 21 months, they suggest that their data show that the introduction of the SAVI device offers more women the opportunity to undergo APBI.
Patients in this cohort ranged in age from 41 to 87 years, with a median age of 60. They had a median tumor size of 1.1 cm, with the largest tumor being 3.5 cm. The majority of patients (65%) had histologically confirmed infiltrating ductal carcinoma, and 31% had ductal carcinoma in situ. After undergoing a lumpectomy, the patients received 3.4 Gy of radiation twice daily, for a total of 34 Gy.
Skin-to-tumor-bed edge ranged from 0.1 mm to 4.4 cm, with a median distance of 1.0 cm. The authors reported that dosimetry was outstanding, with a median percent of target volume receiving 90% of the prescription dose (V90), volume of target receiving 150% of the prescription dose (V150) of 27.8 cm3, and volume of target receiving 200% of the prescription dose (V200) of 14.0 cm3. The maximum median skin dose was 255 cGy per fraction, and for patients with skin spacing of less than 7 mm, it was 280 cGy per fraction.
For patients with a skin bridge of less than 7 mm, 5% had a skin bridge of less than 3 mm. For patients with both chest wall and skin of less than 7 mm, the maximum median lung dose was 205 cGy with simultaneous skin dose of 272 cGy.
Few patients developed toxicities. Sixteen patients developed fibrosis (two grade 2), and 10 patients developed grade 1 hyperpigmentation. Two patients developed telangiectasia, and two patients developed symptomatic seromas, each for an incidence of 1.9%.
Cancer recurrence occurred with one patient. "We believe that the control rate of cancer is very promising," Yashar wrote.
By Cynthia E. Keen
AuntMinnie.com staff writer
July 16, 2010
Related Reading
Long-term APBI outcomes are comparable in low-risk patients, May 31, 2010
New studies hint that APBI could be used in more patients, December 22, 2009
Patients develop moderate distortion after breast cancer brachytherapy, December 2, 2009
ASTRO publishes APBI guidelines for early-stage breast cancer, July 16, 2009
Single-entry APBI offers flexibility in treating breast cancer, April 3, 2009
Copyright © 2010 AuntMinnie.com