A computer model derived from amyloid PET scans appears to be able to predict how early Alzheimer's disease will progress in patients based on distinct brain patterns, according to a study published February 2 in Translational Psychiatry.
Researchers led by Yuqing Sun, PhD, of Beijing Normal University, used an artificial intelligence (AI) algorithm to identify potential patterns of amyloid plaque deposits appearing over time in early Alzheimer's disease patients. Two patterns stood out, and the model could be used to identify patients who may benefit from clinical trials, the authors suggest.
"Our findings suggest that the spatiotemporal variants of amyloid depositions are in close association with disease trajectories," the group wrote. "These findings may provide insight into the disease monitoring and enrollment of therapeutic trials."
The accumulation of amyloid plaque deposits (a hallmark of Alzheimer's disease) affects different brain regions at different time points, and it produces abundant spatiotemporal variations visible on brain PET imaging. Characterizing this spread could enable earlier detection in patients, according to the authors.
To that end, the researchers first identified multiple Alzheimer's disease progression patterns on PET imaging based on variations in brain amyloid deposits. They used the Subtype and Stage Inference (SuStaIn) model, an AI algorithm developed in 2018 that enables the identification of subgroups within cross-sectional datasets.
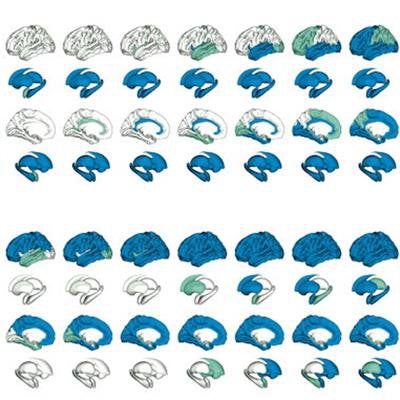
 Regional progression patterns of amyloid accumulation. Green shows the abnormal regions in the numbered stage and blue indicates abnormal regions from the previous stage. The last stage, cerebellum, is not shown here. In the subcortex-priority subtype, amyloid abnormality begins in several subcortex regions and successively affects the cingulate, amygdala, and insula, as well as the cortical regions. In the cortex-priority subtype, the cingulate is the first abnormal region, followed by cortical areas, and then the insula and subcortical regions. Image courtesy of Translational Psychiatry through CC BY 4.0.
Regional progression patterns of amyloid accumulation. Green shows the abnormal regions in the numbered stage and blue indicates abnormal regions from the previous stage. The last stage, cerebellum, is not shown here. In the subcortex-priority subtype, amyloid abnormality begins in several subcortex regions and successively affects the cingulate, amygdala, and insula, as well as the cortical regions. In the cortex-priority subtype, the cingulate is the first abnormal region, followed by cortical areas, and then the insula and subcortical regions. Image courtesy of Translational Psychiatry through CC BY 4.0.Data included amyloid PET scans of 548 and 348 patients who were either cognitively normal or who had mild cognitive impairment. Patients had participated in previous trials through the Alzheimer's Disease Neuroimaging Initiative (ADNI), an international consortium of universities and medical centers.
Based on the SuStaIn analysis, two patterns stood out and were evident in a broad range of individuals. The patterns start with different regions and finally progress to the cerebellum via different pathways.
In subtype 1, which the authors termed the "subcortex-priority subtype," the subcortical regions, followed by the cingulate, and then the insula became abnormal earlier than the cortical areas. In subtype 2, referred to as the "cortex-priority subtype," the cingulate was the first abnormal region followed by the cortical regions, then the insula, and from there the subcortical regions, the authors explained.
Next, the researchers compared these two subtypes and assessed their stages over six years with respect to clinical, genetic, and biomarker characteristics that are known to be associated with Alzheimer's disease. Specifically, they found significant progressive associations between the two amyloid PET patterns and the Mini-Mental State Examination, memory, executive function, language function, visuospatial function, apolipoprotein E gene status, cerebrospinal fluid amyloid, total tau, and phosphorylated tau.
Finally, the researchers validated the results in the validation dataset.
"We applied an unsupervised data-driven method in a broad range of [cognitively normal] and [mildly cognitively impaired] individuals to evaluate the association between spatiotemporal variability in amyloid deposition and [Alzheimer's disease] profiles," the authors wrote. "We identified two reproducible subtypes characterized by different regional progression patterns in two independent datasets."
Ultimately, few studies have examined the association between spatiotemporal variations in brain amyloid deposits and clinical presentation in early Alzheimer's disease patients, and the results will need to be replicated in other cohorts, the authors wrote.
"Our findings highlight the importance of uncovering the spatiotemporal variations of amyloid deposition," Sun and colleagues concluded.