The U.S. Food and Drug Administration has cleared the Artis icono floor ceiling-suspended angiography system from Siemens Healthineers.
Originally introduced in 2019 as floor-mounted angiography systems, the Artis icono line is now available with a ceiling-suspended C-arm for additional positioning flexibility. The new system is designed for both routine and advanced interventional radiology and cardiovascular procedures.
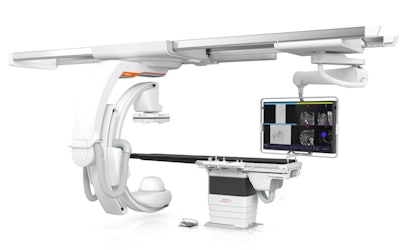 The Artis icono floor ceiling-suspended angiography system from Siemens Healthineers.
The Artis icono floor ceiling-suspended angiography system from Siemens Healthineers.The new version of Artis icono include rotational capabilities and simplified cabling for the system's C-arm; this permits conebeam CT data acquisitions in just 2.5 seconds at the patient's head and 4 seconds at the left and right sides of the patient's body. Such a short 3D spin time reduces motion artifacts and requires less contrast media, according to Siemens Healthineers.
Artis icon also employs the company's Optiq image chain, which allows clinicians to reduce radiation dose to patients while maintaining consistent image quality. Users can set the image quality they desire, and then Optiq sets exposure parameters to meet these criteria.