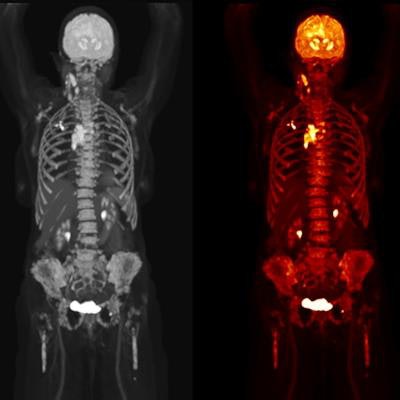
Siemens Healthineers has received clearance from the U.S. Food and Drug Administration for Biograph Vision Quadra, a new PET/CT scanner with a large 106-cm axial field of view.
Initially launched at the 2020 European Association of Nuclear Medicine (EANM) meeting, Biograph Vision Quadra is able to perform simultaneous whole-body imaging from the top of the head to the thigh. The system's 106-cm axial field of view is four times that of Siemens' Biograph Vision 600 scanner.
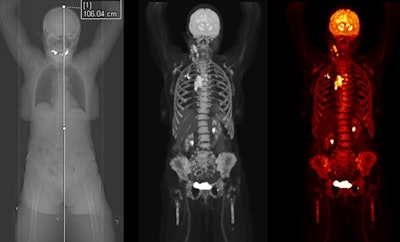 Biograph Quadra Vision can cover more patient anatomy in a single bed position than a standard PET/CT scanner. Image courtesy of Siemens Healthineers.
Biograph Quadra Vision can cover more patient anatomy in a single bed position than a standard PET/CT scanner. Image courtesy of Siemens Healthineers.The new system is designed for clinical use as well as translational research, such as creating therapies and new clinical procedures. Siemens is also highlighting the scanner's 3.2-mm silicon photomultiplier (SiPM) detector technology and time of flight (ToF) performance, which enable more anatomical coverage of a patient in one bed position than a standard PET/CT scanner, according to Siemens.
Finally, Biograph Vision Quadra can be sited in the same-sized space as a conventional PET/CT system.