Echocardiography image analysis software developer DiA Imaging Analysis has received U.S. Food and Drug Administration (FDA) 510(k) clearance and the European CE Mark for use of its artificial intelligence (AI)-based ultrasound algorithms for patients with COVID-19.
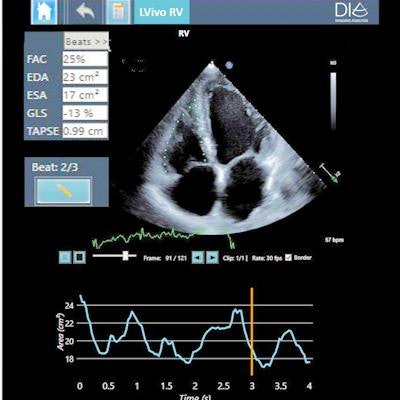
LVivo RV is an AI-based algorithm for automated analysis of the heart's right ventricle and can be used to diagnose and monitor right heart ailments in acute and chronic patients, including those with COVID-19. LVivo Bladder uses AI to deliver automated bladder volume measurements on ultrasound devices, which minimizes scan time and infection risk, according to DiA.
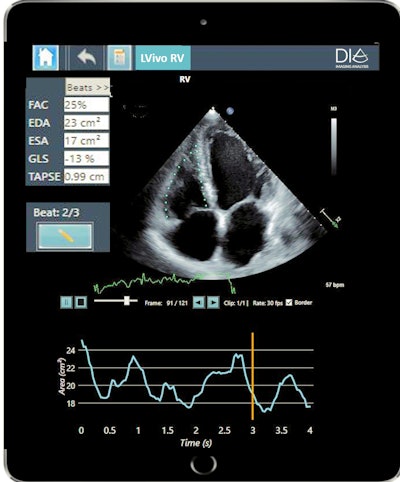 DiA's new AI-based ultrasound analysis tool LVivo RV. Image courtesy of DiA.
DiA's new AI-based ultrasound analysis tool LVivo RV. Image courtesy of DiA.Both algorithms are available as part of DiA's LVivo Toolbox, which now includes six FDA/CE-cleared AI-powered ultrasound algorithms. What's more, LVivo Bladder extends DiA's range of applications from its core market of cardiac ultrasound into abdominal imaging.



















