Top Story
Latest News
More from AuntMinnie
Accuray opens training facility in Switzerland
April 18, 2024
Lumicell's Lumisight, LumiSytem get FDA nods
April 18, 2024
Radiology Leadership Institute names award recipients
April 18, 2024
GE HealthCare launches two new ultrasound systems
April 18, 2024
AdvaMed urges Congress prioritize R&D tax credit
April 17, 2024
Swoop system tested in Alzheimer’s patients
April 17, 2024
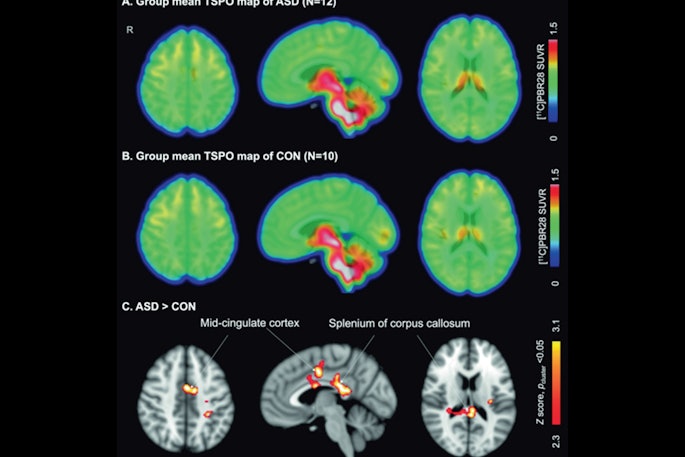
PET tracer for gliomas under expedited review
April 17, 2024
Matsumoto named chair of ACR Board of Chancellors
April 16, 2024