Top Story
Latest News
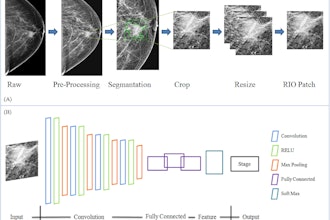
FAPI-PET shows promise in patients with lung cancer
April 15, 2024
IMV: Nuclear medicine procedure volumes decrease
April 15, 2024
Cases of the Week
Check out our Cases of the Week!
More from AuntMinnie
SIR publishes statement regarding pediatric trauma care
April 15, 2024
Flywheel CEO to step down
April 15, 2024
Radiologists experience modest pay increase
April 12, 2024
RadNet teams up with basketball icon Sheryl Swoopes
April 12, 2024
16 Bit gets FDA nod for AI x-ray software
April 12, 2024
Bayer and Hologic collaborate on CEM
April 12, 2024