Top Story
Latest News
More from AuntMinnie
AdvaMed urges Congress prioritize R&D tax credit
April 17, 2024
Swoop system tested in Alzheimer’s patients
April 17, 2024
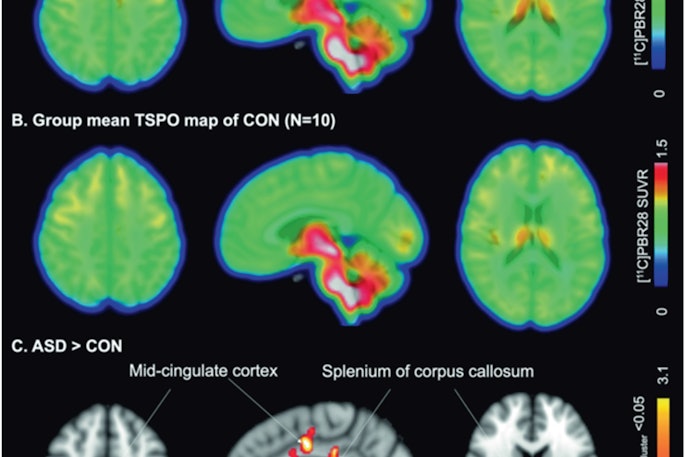
PET tracer for gliomas under expedited review
April 17, 2024
Matsumoto named chair of ACR Board of Chancellors
April 16, 2024
SNMMI declares win in Washington, DC
April 16, 2024
ACR lays off 11% of workforce
April 16, 2024