Top Story
Latest News
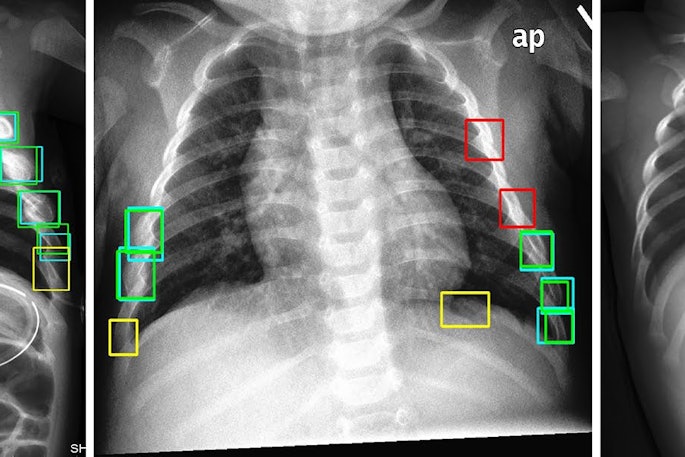
Cases of the Week
Check out our Cases of the Week!
More from AuntMinnie
Matsumoto named chair of ACR Board of Chancellors
April 16, 2024
SNMMI declares win in Washington, DC
April 16, 2024
ACR lays off 11% of workforce
April 16, 2024
SIR publishes statement regarding pediatric trauma care
April 15, 2024
Flywheel CEO to step down
April 15, 2024
Sonio taps new scientific advisory board member
April 15, 2024