CHICAGO - Philips Healthcare is launching a variety of new imaging technologies at this week's RSNA 2018 meeting, including an MRI scanner that dramatically cuts helium use and an updated platform designed to foster the development of artificial intelligence (AI) algorithms.
MRI
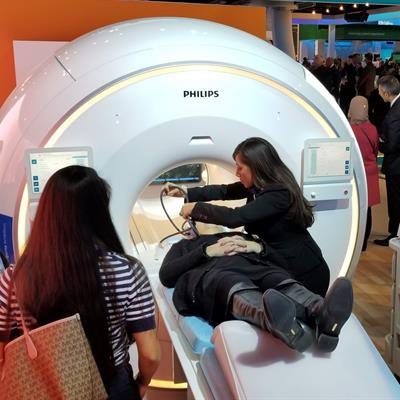
Ingenia Ambition is a new 1.5-tesla system that uses Philips' BlueSeal technology in a fully sealed magnet that dramatically reduces helium consumption. While most MRI scanners use about 1,500 L of helium, Ambition begins with only 7 L and never requires refilling; it also has no quench pipe, according to the company.
 Philips' new Ingenia Ambition 1.5-tesla scanner.
Philips' new Ingenia Ambition 1.5-tesla scanner.The scanner is also 900 kg lighter than its predecessor system in the Philips catalog. The scanner's Easy Switch technology allows the magnet to be ramped down quickly in case of an emergency, Philips said.
First shown at the International Society for Magnetic Resonance in Medicine (ISMRM) meeting in June, Ambition includes the company's Compressed SENSE advanced acceleration application that reduces exam times by up to 50%. Compressed SENSE is also now available on other Philips MRI scanners.
The first U.S. installations of Ingenia Ambition are beginning now, and installations in Europe began in August.
Philips is also highlighting research showing the efficiency challenges that imaging facilities face in using MRI. Results from a new survey show that patient motion and anxiety are major concerns, as these often result in the need for follow-up scans and an increased time to diagnosis.
The survey results indicate that better approaches to managing patient motion and stress are needed, and Philips is highlighting the role that its Ambient Experience and in-bore Connect audiovisual system can play in calming patients. A hospital in Denmark was able to reduce rescans by up to 70% using Ambient Experience in-bore Connect with its 3-tesla magnet, the company said.
Imaging informatics and AI
Another main feature in the Philips booth is IntelliSpace Discovery 3.0, a new software platform designed to facilitate the development and deployment of radiology artificial intelligence (AI) algorithms for clinical and translational research.
The release adds a range of new AI capabilities to the IntelliSpace Discovery platform, which has previously been used to develop radiology applications for image rendering, segmentation, and quantification tasks. Version 3.0 of IntelliSpace Discovery brings new AI-oriented applications and tools designed for radiologists to aggregate, normalize, and anonymize data that can be visualized and annotated for training and validating deep-learning algorithms.
IntelliSpace Discovery 3.0 supports integration with existing clinical infrastructure, enabling easy access to vendor-agnostic and multimodality datasets, according to Philips. In addition, its advanced visualization tools allow image data to be prepared and processed for AI training.
The company is also showing its IntelliSpace Enterprise Edition PACS software with a new Performance Bridge addition that offers users insights into their operations with a dashboard-style interface. The tool is designed to help imaging facilities improve their workflow efficiency and continuously improve their operations.
In addition, Philips has begun making the first installations of its Illumeo artificial intelligence platform, which first debuted at RSNA 2016. The company has been working on integrating Illumeo's AI tools within the workflow of the PACS reading environment, and at RSNA 2018 it is showing a patient avatar that uses natural language processing to analyze patient data and highlight important findings.
Finally, Philips has added new features to its IntelliSpace PACS Advanced Mammography, including dose report PDFs, JPEG pathology information, and the ability for users to set shortcuts for repeated tasks.
X-ray
DigitalDiagnost C90 is a new ceiling-suspended digital radiography (DR) system that includes a tube head with a touchscreen display designed to improve workflow and efficiency. The display shows images on a live camera mounted on the tube head that can help with patient positioning by providing a clear view of the collimated area; this can help alleviate potential imprecise collimation such as with bariatric patients.
 The DigitalDiagnost C90 digital radiography system.
The DigitalDiagnost C90 digital radiography system.C90 uses Philips' Eleva user interface, a common platform across the company's DR systems that is easy to learn and customizable, according to Philips. The DR system also includes Variofocus x-ray tubes, fixed and wireless detectors, and Philips' Unique 2 image processing, as well as a 35 x 43-cm DR panel in a fixed or wireless configuration.
C90 is pending U.S. Food and Drug Administration (FDA) clearance; it is being shipped in Europe.
Ultrasound
On the ultrasound side, Philips is showing its "ultimate ultrasound solution" for breast assessment, available with its Epiq and Affiniti systems, the company said. The package is a combination of Philips' PureWave eL18-4 ultrabroadband linear array transducer, which performs both shear-wave and strain elastography, and anatomical intelligence software, which allows users to map the entire breast. The system includes a specially designed mattress and tabletop field generator.
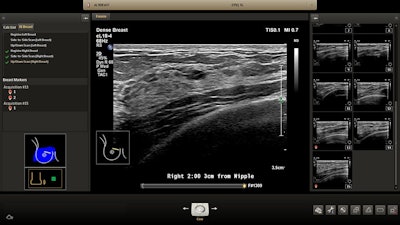 Philips' ultrasound for breast assessment. Image courtesy of Philips Healthcare.
Philips' ultrasound for breast assessment. Image courtesy of Philips Healthcare.The company is also highlighting its "ultimate liver solution," also for the Epiq system, which features shear-wave imaging capability; PureWave transducers; streamlined fusion of CT, MR, and PET images with ultrasound images; and contrast-enhanced ultrasound.
Finally, Philips is touting its Lumify HIPAA-compliant handheld ultrasound platform for mobile devices. It can be used at the point of care and also as a teleradiology tool for educational or global health purposes, the company said.
CT
In the CT section of its booth, Philips is discussing the economic benefits of spectral CT imaging with its IQon Elite scanners. The company is highlighting data that show how the technology can increase hospital efficiency by reducing the number of follow-up scans, while also making it possible for some patients to receive less contrast and x-ray radiation dose while accelerating the time to diagnosis.
Philips is also discussing a new cybersecurity software update that is due to be released in the fourth quarter of 2019. The release will include security enhancements for the company's iPatient application for radiation dose management and workflow. The update will be available for CT scanners across the company's product line.
Molecular imaging
At this year's meeting, Philips continues to highlight its Vereos PET/CT system, which it began shipping in September 2017. The company has installed 65 systems since it launched the device. Vereos features Philips' digital photon-counting technology, which doubles the volumetric resolution, sensitivity, and accuracy of the exam, compared with PET/CT scanners based on conventional photomultiplier technology, according to the firm.
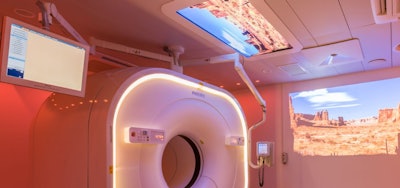 Philips' Vereos PET/CT system. Image courtesy of Philips Healthcare.
Philips' Vereos PET/CT system. Image courtesy of Philips Healthcare.Vereos' three key attributes are that it visualizes small lesions, reduces scan time, and cuts radiation dose, said Piotr Maniawski, Philips' director of clinical science for nuclear medicine. Its Ambient Experience feature eases patient anxiety, which translates into improved image quality, as patient stress can artificially boost FDG uptake, he said.


















