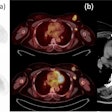
A biomarker blood test can accurately determine if patients with oropharyngeal squamous cell carcinoma (OPSCC) related to human papillomavirus (HPV) infection are free from cancer following radiation therapy, according to research presented at this week's American Society for Radiation Oncology (ASTRO) meeting in San Antonio.
Researchers at the University of North Carolina at Chapel Hill developed a liquid biopsy test that measures fragments of DNA shed by cancer cells in the blood. In a trial, the test was found to be highly sensitive and specific -- including a 100% negative predictive value -- for HPV-associated OPSCC. The researchers believe it could reduce the need for radiological studies such as PET/CT scans during surveillance after radiation therapy.
"This blood test had exceptional performance in monitoring patients for cancer recurrence after radiation therapy," said Dr. Bhisham Chera in a statement from ASTRO. "If the circulating tumor HPV DNA is undetectable, there is a high likelihood that the patient is in remission and cancer-free."
The liquid biopsy test developed by Chera and his colleagues to monitor patients following radiation therapy is a digital polymerase chain reaction assay for HPV DNA. It does not detect other types of cellular DNA, and it can detect as few as six molecules of HPV DNA in a blood sample, according to the researchers.
They evaluated the liquid biopsy test in a prospective trial that included 89 patients with HPV-associated OPSCC whose cancer had not metastasized to other organs. All patients received definitive chemoradiation therapy, and they were monitored -- beginning three months after treatment completion -- with a combination of PET/CT, CT, and chest x-ray studies every six months. Patients also received clinical exams every two to four months for two years, and every six months for the following three years.
Blood was also drawn and tested for traces of the plasma-circulating HPV DNA (ctHPVDNA) during each follow-up visit. Additional imaging tests were performed if ctHPVDNA was detected.
Of the 89 patients, 70 had undetectable levels of ctHPVDNA at every follow-up visit; none showed any sign of cancer recurrence. The remaining 19 patients had a positive ctHPVDNA test result with a median interval from therapy of 16.7 months and a median value of 75 copies/mL. Eight of these patients developed a positive test result and were diagnosed with cancer recurrence, including one regional and seven distant cancers. Meanwhile, the other 11 patients showed detectable levels of ctHPVDNA but no other evidence of cancer recurrence. These patients continue to be monitored with repeat blood tests and imaging, according to the researchers.
Chera said the blood test could provide patients with peace of mind.
"It detects cancer before the scan detects cancer," he said. "Using this test, I can walk into a patient's room and say, 'You are more than likely cancer-free at this point.' "