The U.S. Food and Drug Administration's (FDA) recent approval of a PET radiotracer that targets lesions positive for prostate-specific membrane antigen (PSMA) is not only a "quantum leap" for the diagnosis of prostate cancer in men but also sets a precedent for academic research.
The FDA granted approval to the University of California, San Francisco (UCSF) and the University of California, Los Angeles (UCLA) for the use of gallium-68 (Ga-68) PSMA-11 PET for prostate cancer patients with suspected metastases, as well as in patients with suspected recurrence based on elevated prostate-specific antigen (PSA) levels.
The agency's action also marks a unique approach to simultaneous new drug application (NDA) approvals and opens the door for wider clinical use of the cancer imaging technique.
"The FDA has never approved two institutions' two NDAs at the same time," said Dr. Thomas Hope, associate professor in residence at UCSF. "The fact that the FDA was so understanding and flexible to allow this is what encouraged and enabled an academic collaboration like this. It is a unique process and infrequently does anything like this ever happen in academia."
Clinical trials
To gain approval, UCLA and UCSF conducted two prospective clinical trials that included a total of 960 men with prostate cancer.
"The initial staging portion of the label includes the majority of patients who have intermediate to high-risk prostate cancer," Hope added. "In low-risk patients who have low-volume disease and low Gleason scores, there is minimal to no role for PSMA-PET. In that setting, MRI, transrectal ultrasound, and biopsies would play a majority role in staging these patients."
PSMAs are proteins that are highly overexpressed on cancer cells in patients with prostate cancer.
"Therefore, they provide an excellent target for imaging of prostate cancer," said Dr. Jeremie Calais, head of clinical research in UCLA's nuclear medicine division. "PSMA-targeting imaging agents are more or less the same; they all perform very well. What matters is their availability and the ability to provide them to the patients."
The FDA’s approval of the NDA should help Ga-68 PSMA-11 PET become more available to patients as an approved medical procedure for clinical use, rather than as a research tool, Calais said. If and when Medicare coverage is granted, the imaging technique then could become “a standard-of-care procedure reimbursed for everyone," he said.
One advantage to Ga-68 is that it can be manufactured onsite with a generator, rather than with a cyclotron. That access is particularly beneficial given its half-life of approximately 68 minutes. On the other hand, while the generator can produce quantities of 50 to 100 mCi, its final output is only approximately 20 mCi, which must be split between patients scheduled for PET imaging.
"You might be able to get up to three patients [imaged in a day]," Hope said. "As your generator decays over time, you get less than 20 mCi per synthesis. At UCSF, we generally schedule two patients per synthesis. We try to do three of those per day with a maximum of six patients per day. We end up with a fairly significant backlog of patients because we cannot keep up with the demand."
One alternative to manufacturing greater amounts of Ga-68 is to create a target on a cyclotron, which potentially could produce as much as 1 Curie of the radiotracer. It is "a little more complicated and takes some work to optimize, but you can make more final product, [perhaps] as much as 45 mCi," Hope added. "In our NDA, we included cyclotron-produced Ga-68 PSMA-11 already approved by the FDA, but it will be much harder for many sites to get a cyclotron set up to make gallium-68."
Helping hands
Over the last four years, the University of Iowa also has been conducting a clinical trial with Ga-68 PSMA-11 PET for prostate cancer, the results of which were shared in parallel with researchers at UCLA and UCSF.
"We got into it not only to pretrain our nuclear medicine physicians to interpret the images, but we also wanted our referring clinicians, urologists, and medical oncologists who dealt with prostate cancer patients to understand how best to use it," said John Sunderland, PhD, associate professor of radiology and director of the university's PET imaging center. "What is special about Ga-68 PSMA is we are getting higher performance and more molecularly specific targeting. PSMA's performance particularly manifests itself in recurrent prostate cancer when PSA starts rising."
This latter-mentioned proficiency is especially beneficial when those PSA readings are low, but increasing nonetheless. Ga-68 PSMA-11 PET has shown a propensity to find previously undiscovered prostate cancer from minimal, but rising, PSA levels.
About six months ago, the University of Iowa changed its clinical trial to an expanded-access investigational new drug (IND) protocol, whereby it can broaden the use of Ga-68 PSMA-11 PET. By doing so, the institution expanded its ability to study patients and PSMA within a less restrictive context. So far, some two dozen prostate cancer patients have been imaged under the revised IND.
Speaking anecdotally, Sunderland said the frequency with which previously unknown prostate cancer has been discovered -- and which was responsible for increases in PSA levels -- has been "stunningly high" at approximately 80%. "That information is what guides the referring physician toward a more effective treatment path."
Image interpretation
As for interpreting Ga-68 PSMA-11 PET results, the technique "does require proper training, but can be considered easy in comparison to other techniques," UCLA's Calais said. "We have shown in some papers that after 30 to 50 images, one can reach a very decent level of expertise in reading. The interreader reproducibility also is very high, because of the very high, black-on-white contrast imaging. It is more clear-cut than other metabolic tracers that show the activity of the cell."
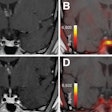
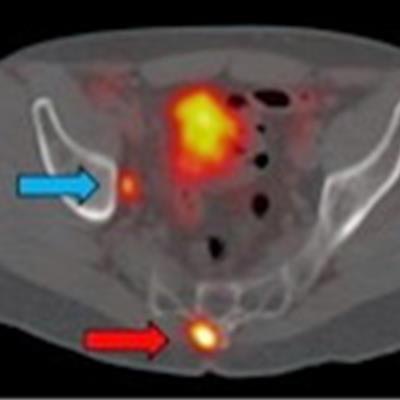
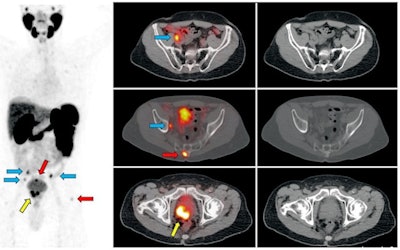 Ga-68 PSMA-11 PET/CT images of a patient with prostate cancer. Conventional imaging was negative for extraprostatic disease spread (metastasis). The PSMA PET/CT image shows prostate cancer lesions. The image enables clinicians to stage the disease as metastatic, therefore enabling the patient to avoid undergoing local therapy alone, which would fail. Yellow arrow shows the primary prostate tumor, blue arrows show the pelvic lymph nodes metastasis, and red arrows show bone metastasis. Images courtesy of Dr. Jeremie Calais and UCLA.
Ga-68 PSMA-11 PET/CT images of a patient with prostate cancer. Conventional imaging was negative for extraprostatic disease spread (metastasis). The PSMA PET/CT image shows prostate cancer lesions. The image enables clinicians to stage the disease as metastatic, therefore enabling the patient to avoid undergoing local therapy alone, which would fail. Yellow arrow shows the primary prostate tumor, blue arrows show the pelvic lymph nodes metastasis, and red arrows show bone metastasis. Images courtesy of Dr. Jeremie Calais and UCLA.To educate its members on Ga-68 PSMA-11 PET, the Society of Nuclear Medicine and Molecular Imaging (SNMMI) has assembled data on the application.
"There is ample information for the nuclear medicine physicians and radiologists to interpret these [images]," added Sunderland, who also serves as vice chair of SNMMI's Research and Discovery Domain. "There is a different physiological uptake pattern and certain things we need to look for in order not to be misled by certain findings. Because it is so specific, this is not a particularly difficult agent to interpret clinically. That being said, we always need to educate our clinicians on the nuances associated with it."
Reimbursement path
Ga-68 PSMA-11 PET still lacks reimbursement coverage, but UCSF's Hope does "not anticipate significant hurdles" for the U.S. Centers for Medicare and Medicaid Services to eventually approve payment, given that the application might be covered under the national coverage decision for oncologic PET imaging, though current procedural terminology codes would need to be requested. Private insurance coverage, however, is "a different story," he said.
In the meantime, prostate cancer patients will have to pay out-of-pocket for Ga-68 PSMA-11 PET imaging.
"We are doing the best we can to keep prices low as possible, so we don't lose money on it, but every place in the near-term -- besides UCLA and UCSF -- will be operating under the auspices of an IND protocol," Sunderland said. "Patients one way or another may have to pay some out-of-pocket for the image."
Achieving Medicare and insurance coverage for Ga-68 PSMA-11 PET would help make it the standard of care to all patients, UCLA's Calais added.
"That could take another six to nine months. Then the next step would be to develop clinical guidelines in which doctors can integrate Ga-68 PSMA-11 PET into their prostate cancer treatment management routines," he said.
Calais and his UCLA colleagues are now conducting trials to determine if Ga-68 PSMA-11 PET can improve the selection of patients with prostate cancer for the most appropriate treatment and thus improving their outcomes.
"We want to show the effectiveness of this individualized approach: the right treatment to the right patients," he said.