Bone Imaging in the Post-operative Patient:
Post-operative
Evaluation of Spinal Fusion:
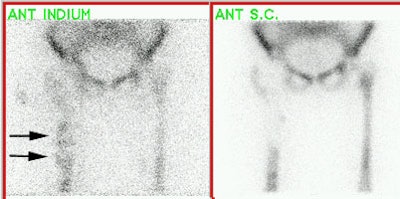
Spinal fusion is performed in patients with back pain due to segmental instability. Persistent back pain in the first few years after the procedure is often related to failure to achieve structural integrity of the fusion (pseudoarthrosis).The incidence of pseudoarthrosis following spinal fusion varies from 10 to 30%, however, not all patients with pseudoarthrosis suffer from back pain. Early failed fusion is usually treated with repeat surgery. Bone scanning can be used to detect the presence of a pseudoarthrosis in patients with continued pain following spinal fusion. On SPECT images successful fusions demonstrate diffuse, but not increased, uptake which can be inhomogeneous, and no evidence of focal abnormalities within the fusion mass. This uptake is probably due to widespread new bone formation. Uptake within the vertebral bodies or apophyseal joints at the fusion levels may be seen and are more common with posterior fusions. These findings may be due to motion within the fusion, despite the absence of pseudoarthrosis. Due to the alterations in impact loading following fusion, focal uptake in the vertebral bodies and apophyseal joints in the free motion segments adjacent to the fusion are commonly seen: 45% of patients following lateral fusion and 87% of patients following posterior fusion (posterior fusion has been shown to produce greater stress in the free segments adjacent to the fusion). A pronounced focal increase in uptake within the fusion mass more than 1 year post-op is highly suspicious for pseudoarthrosis. Generally, significant healing of the fusion is complete by 6 months, but as at other sites of surgery, scintigraphy may remain positive for up to 2 years. As a general rule if healing is taking place, the activity seen within the fusion should decrease in size and intensity. Focal abnormalities within the spinal fusion mass have also been identified in asymptomatic patients, and may represent painless pseudoarthrosis. Late pseudoarthrosis (over 4 years old) may not demonstrate increased tracer localization and this may be related to decreased osteoblastic activity. These lesions, although present, may not be clinically significant as they do not alter skeletal metabolic activity. Asymmetric SI joint activity is also noted frequently in patients that have undergone spinal fusion (up to 75% of cases), with the side of the bone graft harvesting showing less activity than the contralateral side. Increase SI joint uptake can also be seen and may be related to alterations in spinal mechanics [1]. Scintigraphy has a sensitivity of 78% and a specificity of 83% in the detection of pseudoarthrosis, which is superior to plain radiography (43% and 50%, respectively). [1-5]
Bone Grafts:
Bone scintigraphy can also be used to evaluate whether a bone graft is viable or non-viable [30]. Vascular patency to the graft is characterized by normal or diffusely increased tracer uptake throughout the graft (increased uptake in the graft predicts an uncomplicated healing course [30]). Focally increased uptake may be seen at the osteotomy sites. When grafts have lost their vascularity they do not concentrate tracer and will appear as photon deficient areas on delayed bone images with reduced flow on the perfusion study (indicative of vascular occlusion which is followed by necrosis and graft failure [30]). It is important to remember that the scan is most useful for predicting vascular patency and osseous metabolic activity ONLY when the scan is performed within one week of surgery (typically between 2-11 days post-op [30]). Scans performed later may be incorrectly interpreted as showing graft viability because of "periosteal creep" (i.e.: new bone deposition on a non-viable graft). Additionally, although tracer uptake is equated with graft viability, this finding has also been described in patients with osteoradionecrosis- a serious complication of radiotherapy for head and neck tumors. Consequently, the presence of increased uptake in patients who have undergone radiotherapy after mandibular reconstruction cannot be automatically equated with graft viability [1].
Joint Prosthesis Imaging:
The are three forms of hip prostheses- a hemiarthroplasty (partial hip replacement) in which the femoral head and neck are replaced, a total hip arthroplasty (THR) which consists of femoral head/neck and acetabular components, and a resurfacing arthroplasty (RA) in which the native femoral neck is preserved [23,27]. A hemiarthroplasty is commonly performed to treat femoral neck fractures or other proximal femur conditions in which the acetabulum is spared [27]. There are two types of hemiarthroplasty devices- a unipolar prosthesis (which consists of a femoral stem with a fixed head that articulates with the native acetabular cartilage), and a bipolar prosthesis (which have a polyethelene lined metal cup into which a small femoral head with an attached stem is locked [27]. Motion with a bipolar prosthesis may occur between the prosthetic head and cup AND also between the cup and acetabulum [27].In a conventional total hip arthroplasty, both the femoral head and the acetabulum are replaced by prosthetic components [27].
The aim of femoral stem positioning in THA is to place the stem in a neutral/anatomic position within the shaft and allow slight anteversion of the neck [23,27]. On an AP x-ray, the tip should be located in the center of the femur [27]. For a RA, the position of the resurfacing femoral stem should be central in the femoral neck on the frontal view (the position seen on the lateral view is thought to be less critical, but the pin should not impinge on the cortex) [23]. Occasionally there is notching of the superior femoral neck that is likely related to surgical trauma [23]. The recommended range for the acetabular cup inclination is 35-55 degrees (30-50 degrees [27]) and for cup anterversion 10-30 degrees [23] (other indicate 5-25 degrees [27]). A vertically oriented cup may result in excessive loading at the edge of the acetabular cup [23]. The prosthetic femoral head should be symetrically seated within the acetabular component (or slightly inferior) [27]. A femoral head located superiorly (even midly) is not normal and indicates polyethylene wear [27].
Prostheses are fixed to bone to prevent unwanted movement [26]. The componenets can be fixed to bone with, or without cement [26]. Implants may be cemented in place using acrylic cements (polymethylmethacrylate) and various additives [27]. The cement is used primarily to fill voids between the bone and the implant, and less as an adhesive [27]. Cementless fixation is initially achieved by press-fitting a slightly oversized component into a prepared cavity and then special surfaces characteristics of the component allow ingrowth of bone [27]. Screws are sometimes used to afix the acetabular component [27].
Lucency around the component or migration of the component are signs of loosening [23]. However, it is common to identify a thin, linear lucent zone at the component cement interface, especially at the proximal lateral aspect of the stem [27]. This finding should be considered normal if stable, but any enlargement is suggestive of loosening [27]. Another common finding is a thin radiolucent band that is less than 2 mm thick and is demarcated by a sclerotic dense line running parallel to the stem along the bone-cement interface [27]. This band results from a reaction between the cement and the adjacent bone, with formation of a fibrous membrane [27]. A similar finding can be seen about cementless protheses, and should be considered njormal if nonprogressive after two years [27]. A "bone pedestal" is a transverse sclerotic line below the tip of the stem bridging the medullary canal [27]. Cortical thickening occuring in the femoral shaft at the level of the distal end of the stem result from stress alterations and reflect successful fixation of the stem [27].
Cemented prostheses are anchored in position with polymethylmethacrylate which fills the spaces between the bone and the prosthesis- this creates a "mantle" around the prosthesis that produices a uniform fit with evenly distributed forces [26]. Tracer uptake on bone scan at the distal tip of the prosthesis is not commonly seen (only about 10% of patients). If identified, it is typically mild. Activity greater than the intensity of the iliac crest seen only on delayed images (i.e.: normal flow and blood pool) is strongly indicative of loosening. If the uptake about the acetabular component of the prosthesis exceeds that of the activity of the iliac crest, this is also indicative of loosening.
Uncemented components rely on a tight or
"presed" fit into bone for initial fixation [26]. Cementless
prostheses become fixed via bony in-growth into a textured or
porous coating applied to the surface of the prosthesis
(permanent fixation is achieved via the ingrowth of trabelcular
bone over 4-6 weeks) [26]. As a result, peripheral tracer uptake
can be present on bone scan for years after placement of a
cementless prosthesis [32].
Normal plain film findings:
The normal acetabular cup has approximately 40? of lateral
inclination (ie: superior tilt relative to a horizontal line,
such as between the ischial tuberosities) and 15? of anteversion
[26].
Normal scintigraphic findings:
Oswald et al. reported on the bone scan findings in uncomplicated porous coated hip arthroplasties [6]. Increased blood flow or focal blood pool abnormality are only rarely seen in uncomplicated prostheses. Diffusely increased blood pool activity within the muscle groups of the ipsilateral leg is seen in up to 40% of patients at some time during their post-operative course and can be seen as late as 24 months post operatively. This may be related to increase muscle usage in patients who were previously less active. Delayed activity at the distal tip of the prosthesis can be seen in all porous prosthesis at some time. Even 2 years post-op, activity could still be identified medially (15%), distally (70%), and laterally (50%). Activity in the medial segment was always less than or equal to lateral segment activity. Analysis of intensity over time demonstrated a clear trend toward stable or decreasing activity within the medial segment. Although this trend is generally observed in the distal and lateral segments as well, activity in these areas may also be noted to increase over time. This activity was not indicative of prosthetic loosening. Increased tracer activity can also be identified about the acetabular component of the prosthesis (present in up to 76% of patients even after two years) [13]. In uncomplicated prostheses, acetabular activity generally remains stable or decreases in intensity over time [13].
Persistently increased uptake of Tc-MDPin heterotopic bone occurs in most patients with hip prostheses. This is in contradistinction to Tc-MDP uptake in heterotopic bone in paraplegic patients which decreases as the bone matures.
Infection is a serious complication of joint replacement and
can occur in 1% to 4% of patients for hip prostheses (the rate
of infection following revision surgery is somewhat higher) [15,17,28]. About one-third of infections
develop within 3 months, another third within one year, and the
remainder develop more than 1 year
after surgery [15]. Staphylococcus epidermidis
and S aureus are the most
common organisms [15]. Patients often present with joint pain
that can also occur in patients with aseptic loosening. False
negative and false positive culture results have been reported
in more than 10% of joint fluid aspirates [28]. Nonspecific
markers of inflammation such as the ESR and C-reactive
protein may also be elevated in both infection and aseptic
loosening [19]. The diagnostic accuracy is best for interleukin
6 [28].
An infected joint prostheses requires a resection arthroplasty amd a thorough debridement, followed by a long course (6 weeks to 3 months) of antibiotics, and a lengthy hospitalization [28]. A temproary cement space laden with antibiotics may be used in the hip joint [28]. Revision arthoplasty is then performed several months later [17]. Differentiation of aseptic loosening from infection is therefore vitally important [15]. Joint aspiration suffers from large numbers of false positive (3-16%) and false negative (up to 10%) results [15,22]. Plain film findings are usually normal (50%) in patients with septic hip prosthesis. Non-focal periprosthetic bone resorption more than 2mm wide is non-specific and can also be seen in loosening.
Conventional bone scintigraphy has a sensitivity of 65%, specificity of 70%, and an accuracy of 50-70% [15] for the diagnosis of infected prosthesis. Oswald et al. reported on the bone scan findings in uncomplicated porous coated hip arthroplasties [19]. Even in patients without loosening or infection, increased tracer activity can seen about the distal tip at 2 years medially (15%), distally (70%), and laterally (50%). With infection, classically, the bone scan is 3-phase positive with diffusely increased periprosthetic activity on delayed images associated with infection (as opposed to loosening in which there is a focal abnormality identified about the prosthesis) [15,17]. The appearance is probably due to generalized osteolysis. Unfortunately, the findings on bone scan are non-specific and there is considerable overlap between infection and loosening [15]. False positive scans can occur due to dystrophic ossification, periprosthetic granulomas, the altered distribution of red marrow, aseptic loosening, damage of the polyethylene surface of the prosthesis, and metallosis. None-the-less, bone scintigraphy is useful as a screening test because a normal scan essentially excludes a prosthetic complication [15].
Labeled WBC imaging can also be used to evaluate for the presence of an infected prosthesis. WBC imaging should be most useful in detecting leukocyte mediated infectious processes- such as an infected prosthesis- and uptake should be absent in loosening (in which the associated inflammatory reaction lacks significant leukocytes) [17]. On average, combined In-111 WBC-bone scintigraphy imaging has a sensitivity of about 67%, and a specificity of 78% for the detection of prosthetic infection. Note that the patients white blood cell count does not correlate with the amount of In-111 WBC uptake and that the patient may have a normal WBC count, even with an infected prosthesis.
Normal post operative In-111 WBC scan findings have been described prior to the use of combined bone marrow scintigraphy and the findings were likely related to bone marrow activity. In-111-WBC uptake was described at the prosthesis tip in 80% of patients at some time, and still present 2 years post-operatively in 50% of patients. Two distinct patterns of WBC uptake were seen identified: Focal (55%) or diffuse linear (45%) activity within the marrow space below the tip of the prosthesis. This uptake demonstrated a clear trend toward becoming stable or decreasing over time in 90% of patients. In 111-WBC activity was also usually less than (32-60%) or equal to (17%) the activity within the iliac crest. Abnormalities with activity greater than that in the iliac crest should be considered suspicious for infection. In comparison to the Tc-MDP scan, the intensity of In 111-WBC uptake at the tip of the prosthesis was less than or equal to the Tc-MDP uptake in 85% of patients (using the iliac crest as a reference). In-111 WBC accumulation also occurs about the acetabular component of the uncomplicated prosthesis in up to 92% of cases and can still be seen even after two years [13]. The activity is always less intense or equal to activity in the iliac crest and typically remains stable or decreases in intensity over time [13]. Mild WBC accumulation can be seen within heterotopic bone and this should not be confused with the presence of infection. Inguinal nodal activity may also be seen- the lateral view is helpful in demonstrating that the abnormality is anterior to the hip. Additionally, patients with heterotopic bone or inguinal nodal activity typically do not demonstrate a flow or blood pool abnormality on their bone scan.
Presently, evaluation of a suspected infected joint prosthesis is best accomplished by combined In-111 WBC- Tc99m sulfur colloid scintigraphy [20]. Localized ectopic bone marrow expansion may occur in the appendicular skeleton after trauma (fracture), surgical interventions- such as joint prosthesis surgery, neuropathic joint, inflammation, or even calvarial hyperostosis [16,20]. Combined leukocyte-marrow imaging can overcome many of the problems created by variable marrow distribution post-operatively and should be used regularly. In leukocyte imaging it is neither the presence nor the intensity of labeled leukocyte activity, but rather the relationship of such uptake to bone marrow activity that is important [15]. Non-specific In-111 WBC accumulation within areas of ectopic bone marrow can be misinterpreted as infection [15]. The addition of Tc-sulfur colloid (Tc-SC) imaging can aid in evaluation of suspected prostetic hip infection by demonstrating normal bone marrow distribution. Any spatially incongruent area of In-111 WBC tracer activity which does not match marrow activity should be considered consistent with infection (regardless of the intensity of the tracer uptake) [15]. When the distributions of the two tracers are spatial congruent, the exam is negative for infection [15]. The intensity of WBC uptake can be misleading, even mild uptake is consistent with infection if it is spatially incongruent with marrow activity. Combined marrow scintigraphy using In-111 WBC's and Tc-sulfur colloid provides the best results for the evaluation of the suspected infected prosthesis with sensitivity, specificity, and accuracy greater than 90% [1,15,17,18,20].
For the exam, In-111 WBC imaging are acquired 24 hours following tracer administration [20]. For the bone marrow exam, patients should be injected with 10 mCi of freshly prepared Tc-SC not more than 2 hours old (older agent may result in persistent blood pool and urinary bladder activity that degrade image quality [20]. The interval between injection and imaging shouild be at least 30 minutes to maximize tracer clearance from the circulation [20]. Simultaneous dual isotope imaging is performed using a medium energy parallel hole collimator with a 10% energy window centered on 140 keV for technetium; and a 5% window centered on 174 keV, and a 15% window centered on 247 keV for In-111 imaging [20]. Images should be acquired for 10 minutes per view with a 128 x 128 matrix [20].
|
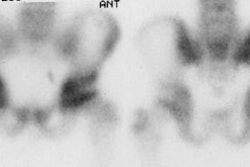
Infected hip prosthesis: The patient shown below underwent evaluation for infected right hip prosthesis using combined In-111 WBC and Tc-sulfur colloid marrow imaging. The white blood cell exam demonstrated incongruent WBC accumulation along the mid and distal portions of the prosthesis (black arrows). Note that other areas of white blood cell accumulation are congruent with the bone marrow scan. The patient had an infected right hip prosthesis. |
|
|
Sequential bone-gallium imaging can also be used to evaluate for prosthesis infection, but the overall accuracy (70-80%) is less than that of combined In-111 WBC - Tc-Marrow scintigraphy [15,17]. For the gallium exam, the study is negative for infection when the gallium scan is normal, regardless of the bone scan findings, or when the spatial distributions of the two tracers are congruent and the intensity of gallium uptake is less than that of the bone tracer [15]. The exam is positive for infection when the distribution of the tow tracers are spatial incongruent or when their distributions are spatial congruent, but the intensity of gallium uptake exceeds that of the bone agent [15]. The images are equivocal for infection when the distributions of the two tracers are spatial congruent and the intensity of uptakes are similar [15].
FDG PET imaging is also being studied for the evaluation of prosthesis infection.
Hip
Prosthesis Loosening:
Aseptic loosening is believed to be cased
by repeated mechanical stress and local host response to the
wear debris of high-denisty
polyethylene, metals, and bone cement [21]. By 10 years after
implantation, 50% of prostheses demonstrate radiographic
evidence of loosening and 30% require revision [15,17]. Aseptic loosening can be treated
with a single-stage revision arthoplasty
requiring only one hospital admission [15,17].
Loosening is being increasingly recognized as the result of an
immune reaction (type IV hypersensitivity) between the patient
and the prosthesis [17]. A metal induced reactive mass can
develop due to a hypersensitivity reaction to metal particles
that have been shed from the prosthesis [23]. Particulate
debris produced by component fragmentation activates tissue
phagocytes, but the debris is resistant to enzymatic destruction
[17]. Continued attempts to phagocytize
the debris results in bony osteolysis
and loosening [17,28]. Histopathologically,
a pseudomembranous structure
develops in the soft tissues at the bone-prosthesis interface
containing mostly histiocytes,
giant cells, lymphocytes, and plasma cells [17]. Aseptic
lymphocytic vasculitis-associated (or lymphocyte dominant)
lesion (ALVAL) is the name that is now commonly applied to the
condition (it has also be referred to as aggressive granulomatosis or granulomatous
pseudotumor) [25]. Metal on metal arthroplasty failure appears as
soft tissue and synovial inflammation, pseudotumor formation,
and osteolysis on imaging and pathologically encompasses the
spectrum between metalosis and ALVAL- these various reactions
have been collectievly termed adverse reaction to
metalic debris (ARMD) [33]. ALVAL
is particularly associated with metal-on-metal total hip
replacements and can be associated with a poor outcome following
revision surgery in patients with advanced disease [24]. Neutrophils are present in less than 10%
of the cases (unlike in infection in which neutrophils
predominate) [17].
Risk factors for developing a reactive mass include female sex
(8:1 greater risk than men), small prosthetic cup size, poor
positioning of the components, and inadvertant downsizing of the
femoral head in women with high preoperative head-neck diameter
ratios [23]. Presening symptoms are often delayed and include
pain, a palpable mass, and femoral neuropathy [23]. The
incidence of reactive mass is variable and depends on the type
of prosthesis used, but can be up to 8% of large femoral head
metal-on-metal THR's [23]. Patients with bilateral RA who
develop a reactive mass in one hip, have a 33% chance of having
a lesion on the other side [23].
Plain film findings which support porous prosthesis loosening include finding a progressive lucent line wider than 2mm adjacent to 3 of the 7 regions about the prosthesis. These findings are felt to represent mechanical loosening. Serial plain film radiographs have sensitivities of 95% and 100% for the detection of loosening of the acetabular and femoral components (respectively) [14].
Unlike aseptic osteolysis, which can be identified as a zone of
lucency around the prosthesis, plain films are generally of
limited value for the detection of aseptic lymphocytic
vasculitis-associated lesion as the films are usually normal
[24]. ALVAL can appear as an area of radiolucency/resorption
of the medial calcar adjacent to the prosthesis usually
without a sclerotic reaction [24,63], that does not conform to
the shape of the prosthesis and can progress over time. Both of
these findings, however, have also been described in association
with infection. The metallosis can sometimes produce a
"cloudlike" radiodensity about the neck of the femoral component
[28].
The masses can be identified on US, CT, and MR [23]. The lesions may have a variety of appearances and appear either cystic or solid [23]. Predominently solid lesions tend to be located anteriroly, usually within the psoas muscle and may extend into the pelvis [23]. Predominantly cystic lesions tend to arise from the posterior joint space [23]. Lateral lesions tend to involve the trochanteric bursa [23].
On bone scintigraphy, focal abnormalities (not diffuse) in two or more
zones or intense uptake in one zone about the prosthesis is
indicative of loosening [14]. For acetabular
loosening, the sensitivity of bone scanning is 97%, and the
specificity is 95% (Accuracy 90%) [14]. For
femoral component loosening the bone scan sensitivity is 85%,
and the specificity 100% (Accuracy 89%) [14]. Therefore, bone
scan does not always provide additional information with regard
to loosening of the hip arthroplasty
compared to serial plain film evaluation [14]. Bone scan is
probably most useful when the radiographs are inconclusive with
regard to loosening [14]. In aseptic loosening there is an
associated inflammatory infiltrate consisting of predominantly
lymphocytes and giant cells (neutrophils
are usually not present in significant numbers). As such,
gallium scintigraphy can
demonstrate increased uptake in aseptic loosening which may be
misinterpreted as infection. In-111 WBC imaging should not be
positive with aseptic loosening due to the absence of
leukocytes. In general, if the plain radiographs do not show
loosening, an aspiration of the hip is negative, and there is no
increased activity on a bone scan, then the hip arthroplasty is neither loose nor
infected [14].
Increased FDG activity is highly sensitive for detection of
periprosthetic granulomatous inflammatory change [63].
MRI with metal artifact reduction sequences can be used to
identify ALVAL [24]. Since magnetic susceptabiloity artifact is
directly proportional to field strength, the use of high-field
strength systems should be avoided [24]. The main finding on MRI
is a periprosthetic soft-tissue mass or fluid collection with a
thick, ragged capsule characteristically extending from the from
the neck of the femoral component [24].
Heterotopic bone formation:
Heterotopic ossification refers to the presence of bone in the soft tissues [29]. Heterotopic new bone formation occurs in 15-50% of patients following hip replacement, but a clinically significant limitation of motion is rare (1-5%) [28]. Predisposing factors include male sex, total cemented prosthesis, lateral surgical approach, infection, post-traumatic arthritis, ankylosing spondylitis, and previous hip surgery [28]. Treatment with low-dose radiation and nonsteroidal antiinflammatory drugs (indomethacin) have been shown to be effective for prophylaxis [28]. Intense uptake is seen on bone scanning when the new bone is immature. Decreasing uptake can be seen as the bone matures. This is important because there is a lower recurrence rate of new heterotopic bone formation if surgery to remove the bone is performed when it has reached maturity.
The incidence of infection after TKA has ranged from 1 to 2% [7,15]. The risk for infection following revision is slightly higher (about 5%) [19]. Aspiration of the knee is helpful, but negative results do not exclude a deep infection [7]. It is even more difficult to assess total knee replacements on bone scan, because 60% of the femoral components and 90% of the tibial components demonstrate increased periprosthetic activity more than 1 year after surgery in asymptomatic patients [1,8]. Periprosthetic activity can persist for several years [17].
White blood cell imaging has a sensitivity, specificity, and accuracy of approximately 85% in the diagnosis of infected knee prosthesis [7]. False positive exams have been reported in association with active rheumatoid arthritis and with massive osteolysis of the adjacent femur and tibia [7].
|
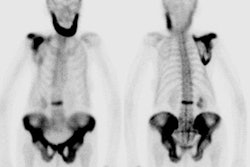
Infected knee prosthesis: The patient had a history of an infected right knee prosthesis. Combined In-111 WBC and Tc-sulfur colloid marrow imaging was performed to evaluate for residual infection following treatment prior to re-do knee replacement. The white blood cell exam demonstrated incongruent WBC accumulation along the mid portion of the right knee joint (black arrow) consistent with unresolved infection. |
|
|
REFERENCES:
(1) Semin Nucl Med 1995; Palestro CJ. Radionuclide imaging after skeletal interventional procedures. 25: 3-14
(2) J Nucl Med 1993; Collier BD, et al. Bone scintigraphy: Part 2. Orthopedic bone scanning. 34(12):2241-6. Review.
(3) J Nucl Med 1989; Lusins JO, et al. Bone SPECT in patients with persistent back pain after lumbar spine surgery. 30: 490-96
(4) J Nucl Med 1994; Even-Sapir E, et al. Assessment of painful late effects of lumbar spinal fusion with SPECT. 35(3):416-22.
(5) Skeletal Radiol 1987; Slizofski WJ, et al. Painful pseudarthrosis following lumbar spinal fusion: detection by combined SPECT and planar bone scintigraphy. 16(2):136-41
(6) J Nucl Med 1989; Oswald SJ, et al. Three-phase bone scan and indium white blood cell scintigraphy following porous coated hip arthroplasty: a prospective study of the prosthetic tip. 30(8):1321-31.
(7) Clinical Orthopaedics and Related Research 1990; Rand JA, Brown ML. The value of indium 111 leukocyte scanning in the evaluaiton of painful or infected total knee arthroplasties. 259: 179-182
(8) Nuclear Medicine Annual 1994, Palestro CJ. Musculoskeletal infection. Ed. Freeman LM. Raven Press, Ltd. New York. 91-119
(9) Radiology 1988; Magnuson JE, et al. In-111-labeled leukocyte scintigraphy in suspected orthopedic prosthesis infection: Comparison with other imaging modalities. 168: 235-239
(10) British Journal of Radiology1992; Miles BKA, et al. Scintigraphic abnormalities in patients with painful hip replacements treated conservatively. 65: 491-494
(11) British Journal of Radiology1992; Copping BC. The role of 99m-Tc-HMPAO white cell imaging in suspected orthopaedic infection. 65: 309-312
(13) J Nucl Med 1990; Oswald SG, et al. The acetabulum: A prospective study of three-phase bone and indium white blood cell scintigraphy following porous-coated hip arthroplasty. 31: 274-280
(14) J Bone Joint Surg [Br] 1993; Lieberman JR, et al. Evaluation of painful hip arthroplasties. Are technetium scans necessary? 75-B: 475-78
(15) Radiographics 2001; Love C, et al. Role of nuclear medicine in diagnosis of the infected joint replacement. 21: 122901238
(16) J Nucl Med 1998; Kaim A, et al. Ectopic hematopoietic bone marrow in the appendicular skeleton after trauma. 39: 1980-1983
(17) J Nucl Med 2003; Palestro CJ. Nuclear medicine, the painful prosthetic joint, and orthopedic infection. 44: 927-929
(18) J Nucl Med 2004; Pelosi E, et al. 99mTc-HMPAO-leukocyte scintigraphy in patients with symptomatic total hip or knee arthroplasty: improved diagnostic accuracy by means of semiquantitative evaluation. 45: 438-444
(19) J Nucl Med 2004; Love C, et al. Diagnosing infection in the failed joint replacement: a comparison of coincidence detection 18F- FDG and 111In-labeled leukocyte/ 99mTc-sulfur colloid marrow imaging. 45: 1864-1871
(20) Radiographics 2006; Palestro CJ, et al. Combined labeled leukocyte and technetium 99m sulfur colloid bone marrow imaging for diagnosing musculoskeltal infection. 26: 859-870
(21) J Nucl Med 2009; Lorberboym M, et al. The use of 99mTc-recombinant human annexin V imaging for differential diagnosis of aseptic loosening and low-grade infection in hip and knee prosthesis. 50: 534-537
(22) AJR 2011; Squire MW, et al. Preoperative diagnosis of periprosthetic joint infection: role of
aspiration. 196: 875-879
(23) AJR 2011; Ostlere S. How to image metal-on-metal
prostheses and their complications. 197: 558-567
(24) AJR 2012; Yanny S, et al. MRI of aseptic lymphocytic
vasculitis-associated lesions in metal-on-metal hip
replacements. 198: 1394-1402
(25) Am J Clin Pathol 2010; Watters TS, et al. Aseptic
lymphocyte-dominated vasculitis associated lesion: a
clinicopathologic review of an unrecognized cause of prosthetic
failure. 134: 886-893
(26) Radiographics 2012; Roth TD, et al. CT of the hip
prosthesis: appearance of components, fixation, and
complicaitons. 32: 1089-1107
(27) AJR 2012; Mulcahy H, Chew FS. Current concepts of hip
arthroplasty for radiologists: Part I, features and radiographic
assessment. 199: 559-569
(28) AJR 2012; Mulcahy H, Chew FS. Current concepts of hip
arthroplasty for radiologists: Part 2, revisions and
complications. 199: 570-580
(29) J Nucl Med 2002; Shehab D, et al. Heterotopic
ossification. 43: 346-353
(30) J Nucl Med 2013; Wong KK, Piert M. Dynamic bone imaging
with 99mTc-labeled diphosphonates and 18F-NaF:
mechanisms and applications. 54: 590-599
(31) Radiographics 2016; White ML, et al. Specturm of benign
articular and periarticular findings at FDG PET/CT. 36: 824-839
(32) AJR 2019; Sethi I, et al.
Current status of molecular imaging of infection: a primer. 213:
300-308
(33) AJR 2021; Petscavage-Thomas JM, Ha A. Best practices: best
imaging modality for surveillance of metal-on-metal hip
arthroplasty. 216: 311-317