OncoScint (Satumomab Pendetide):
General:
Chemistry and Pharmacology:
Oncoscint is an IgG murine monoclonal antibody (B72.3) that specifically targets the cell surface mucin-like glycoprotein antigen TAG-72 which is commonly found on colorectal and ovarian carcinomas. Oncoscint is reported to be reactive with 83% of colorectal carcinomas and 97% of ovarian carcinomas. There is only minimal cross reactivity with other tumors or normal tissues (false-positive rate is very low), but can be seen with mucin-secreting epithelial tissues such as salivary gland ducts, post-ovulatory endometria, certain benign ovarian tumors, and fetal gastrointestinal tissue. Tumor detection is significantly higher in patients with positive TAG-72 serum levels, than those with normal TAG-72 levels. The performance of the agent is also improved whenever the percentage of tumor cells expressing TAG-72 is 40% or greater. A major advantage of Oncoscint is that it allows one to survey the entire body, thus permitting the detection of occult mets which can have a major impact on tumor staging.
Oncoscint is labeled to Indium-111 chloride. The physical half-life of Indium (67 hours) is suitable for imaging intact IgG which usually has a biologic half life of between 30 and 60 hours. One milligram of monoclonal antibody (MoAb) labeled to 5 mCi of In-111 is given via slow I.V. infusion over 5 minutes. Vital signs should be obtained before and 15 minutes following the infusion. Planar 10 minute images can be obtained between 48 and 120 hours post injection. SPECT imaging has been shown to increase lesion detection and should be performed of at least the abdomen and pelvis.
Distribution:
Activity is normally seen in the liver, spleen, and to a lesser extent in the spine and other areas of bone marrow. Male gonadal activity is also usually seen and nipple activity can be seen in women of child bearing age. Variable amounts of large bowel activity is also frequently present and a bowel prep before imaging is recommended. Liver uptake is about 10-15% of the injected dose and this activity can hinder the ability to visualize liver mets- the liver is the site of IgG metabolism and free In-111 also localizes to the liver. Kidney and bladder activity can uncommonly be seen. The critical organ is the spleen which receives a dose of about 16 rads. But the liver (15 rads) and bone marrow (12 rads) also recieve large doses.
Non-pathologic uptake of the tracer has also been seen in:
- Uptake in the surgical scar can be seen up to 6 weeks post-operatively. Imaging prior to this time is generally not recommended so as to reduce non-specific accumulation of tracer at sites of inflammation and surgical wounds.
- Non-pathologic recent fracture (probably related to local hyperemia).
- Aortic aneurysm.
- Uptake at the tip of a central venous catheter.
- Variable activity at ostomy sites: These sites should be marked on the film.
- Severe localized degenerative changes of the spine and sites of degenerative arthritis.
- Discitis.
- Sites of previous intramuscular injections.
- Axillary nodal activity can be seen in association with infiltration of the radiotracer during injection.
- Prior bone XRT will produce decreased marrow accumulation of the tracer and external attenuators may produce cold lesions in the liver.
Indications for the Oncoscint exam:
1. Post-operative evaluation of local recurrence vs. post-op/XRT changes:
Up to one-third of patients with recurrences do not have an elevated CEA [Annual 93, p.14]. In general, a slowly rising CEA indicates a local or regional recurrence, while a rapidly rising CEA is associated with liver mets.
2. Detect occult metastases or synchronous lesions
3. Define the extent of regional disease:
The number and location of cancer positive lymph nodes found in mesenteric tissue following resection for colorectal carcinoma plays an important role in patient staging and prognosis. Survival rates of up to 80% have been described in node negative patients. When one to 4 nodes are involved, 5 year survival drops to 25%, and to 10% if 5 or more nodes are involved. Hand held probes may aid in the detection of mets to small, otherwise normal appearing lymph nodes [JNM, Oct. 93, p.1818].
4. Follow-up of patients at high risk for recurrence:
Dukes B2, B3, C
5. Confirm the presence of malignancy when a biopsy reveals severe dysplasia or atypia.
Colorectal carcinoma:
Patients with primary colorectal carcinoma undergo an extensive preoperative staging work-up. Unfortunately, the accuracy of non-surgical staging techniques (CT or MRI) is poor. Oncoscint has the advantage of permitting whole body screening.
Oncoscint has an overall detection rate (sensitivity) of about 70% for colorectal carcinoma, a specificity of 75%, a positive predictive value of 97%, and a negative predictive value 20% (the high PPV and low NPV may be related to the high incidence of carcinoma in the patients referred for the exam during clinical trials). These numbers are similar to those for CT and the sensitivity of both modalities is limited for small lesions (less than 2 cm). Immunoscintigraphy and CT, however, are actually complimentary, and their combined use increases the per patient sensitivity to 88.4%. Additionally, the results of the Oncoscint exam resulted in a change in patient management in 25% of cases, and the exam also detected sites of occult disease in 10% of patients.
Recurrent Disease:
Recurrent disease is seen in up to 40% of patients with Dukes stage B (2,3) or C colorectal carcinoma, generally within the first 18 months post-op. CEA levels are used to monitor patients for recurrent disease, unfortunately, almost one-third of patients with recurrence do not have elevated CEA levels. Recurrence is limited to a single site in 75% of cases, most commonly the liver (33%) or lung (22%), but local (20%) or intra-abdominal (18%) recurrences are also common. Recurrence in retroperitoneal lymph nodes is seen in about 10% of patients. Resection of an isolated hepatic or pulmonary recurrence can result in survival rates of 33% and 25%, respectively. Both CT and MRI are inadequate in the detection of local or lymph node recurrence. Oncoscint can be used to confirm the absence of other sites of disease prior to surgery.
Liver Mets:
In the evaluation of colorectal carcinoma, the identification of liver mets is very important as many series have a shown a 20-40% 5 year survival rate after resection of isolated colorectal liver mets. Therefore prior to undertaking liver resection, it is important to exclude the presence of disease elsewhere. CT scans have a higher sensitivity for the detection of liver lesions than does Oncoscint (85% vs. 40 to 55% for Oncoscint). In the liver, lesions larger than 2.5 cm, poorly differentiated lesions, or lesions with more than 50% necrosis usually appeared as cold defects. Lesions less than 2.0 cm, well differentiated lesions, or lesions with less than 20% necrosis tended to appear as hot spots or areas of positive radiotracer localization (this may represent a transition period from a hot to a cold lesion). The use of SPECT is important for proper evaluation of liver mets. SPECT detected liver mets in 14% of patients with negative planar images. Combined Tc-sulfur colloid and Oncoscint SPECT studies increases the number of detectable liver lesions by 30%.
Extrahepatic Abdominal Mets:
In clinical trials, OncoScint appeared to be more sensitive than CT in the detection of pelvic or extrahepatic abdominal colorectal carcinoma. Immunoscintigraphy identified 74% of pelvic tumors (vs. 57% by CT) and extrahepatic abdominal sites of disease in 84% of cases (vs. 41% by CT).
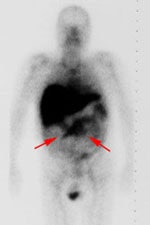
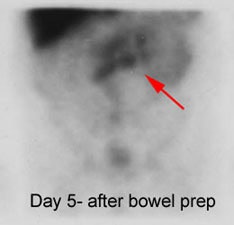
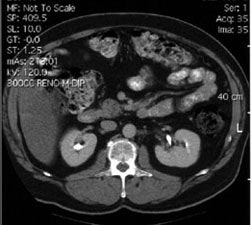
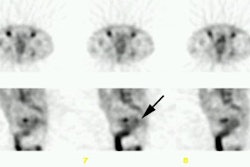
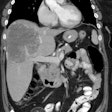
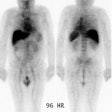
Recurrent colorectal carcinoma: The patient shown below had a history of colon cancer and was being evaluated for a rising CEA-level. CT scan had been interpreted as negative. The OncoScint exam demonstrated focal abnormal tracer activity within the mid-abdomen (red arrows). This activity persistent on delayed images following administration of a bowel prep. At surgery the patient was found to have omental disease and involved lymph nodes corresponding to the abnormality on the OncoScint exam. Even in retrospect, the CT images did not demonstrate definitive abnormality (Click CT for more images) |
|
Ovarian Carcinoma Imaging:
Epithelial cancers are the most common ovarian malignancies and most patients (70%) present with advanced disease. Ovarian cancer in the 4th leading cause of cancer deaths in women. Serum CA-125 levels (a tumor associated antigen found on the surface of ovarian cancers) are useful in predicting the presence of disease, but negative titers do not preclude malignancy. The TAG-72 antigen has been demonstrated in 70-90% of ovarian tumors. In clinical trials, OncoScint had sensitivity of 70% versus 44% for CT, and a specificity of 55% (79% for CT) in patients with ovarian cancer. Carcinomatosis was detectable by antibody imaging in 71% of patients, but in only 45% by CT (Carcinomatosis generally appears as a generalized increase in tracer activity over the abdomen and pelvis which obscures the normally visualized iliac vessels). Occult lesions were detected in 28% of patients, and patient management was altered in 27%. In the evaluation of recurrent ovarian carcinoma it detected more sites of disease than CT and had a sensitivity of 60%, specificity of 60%, positive predictive value of 83%, and a negative predictive value of 30%.
Complications/Side Effects:
Side effects are seen in less than 4% of patients and are generally minor and transient such as fever/chills (2%), rash/pruritis, hypotension, and headache. Infrequent reactions include chest pain, angioedema, or temporary joint pain. Potentially serious reactions include: Anaphylaxis (although not seen in clinical trials), serum sickness with fever, urticaria, arthalgias, or renal dysfunction. Repeat injection of the tracer (in HAMA negative patients) is associated with a similar adverse reaction rate (2 to 4%).
Caution should be exercised in patients who have previously received murine based antibody products. Premedication with prednisone and benedryl is recommended in such cases. Care should also be taken in patients with COPD who may be at a slightly increased risk of bronchospasm.
HAMA Formation:
Administration can result in the formation of human anti-murine antibodies (HAMA) which are found in up to 40% of patients after the examination. HAMA levels became negative (undetectable or less than 400ng/ml) in about half of these patients after 4 to 12 months. The presence of HAMA can alter the clearance and biodistribution of MAbs. Rapid uptake of the immunoconjugates in the liver may occur. Additionally, the risk of potentially serious side effects may be increased, although no correlation between the presence of HAMA and adverse reactions has yet been demonstrated. The presence of HAMA also generally results in falsely elevated levels of CEA and CA125 on in-vitro immunoassays. Non-murine immunoassays may need to be used in these patients.
In HAMA positive patients there is increased concentration of the agent in the liver with diminished blood pool activity.
REFERENCES:
(1) Radiology 1990; Abdel-Nabi H, et al. In-111-labeled monoclonal antibody immunoscintigraphy in colorectal carcinoma: Safety, sensitivity, and preliminary clinical results. 175: 163-171
(2) Radiology 1992; Collier BD, et al. Immunoscintigraphy performed with In-111-labeled CYT-103 in the management of colorectal cancer: Comparison with CT. 185: 179-186
(3) Semin Nucl Med 1993; Abdel-Nabi H, Doerr RJ. Clinical applications of indium-111-labeled monoclonal antibdoy imaging in colorectal cancer patients. 23: 99-113
(4) J Nucl Med Technol 1993; Trembath LA, et al. A practical approach to monoclonal antibody imaging. 21: 171-176
(5) Gynecologic oncology 1992; Surwit EA, et al. Clinical assessment of 111In-CYT-103 immunoscintigraphy in ovarian cancer. 48: 285-292