The U.S. Food and Drug Administration (FDA) announced on Friday the first regulatory clearance of a hybrid PET/MRI scanner in the U.S., with Siemens Healthcare receiving 510(k) clearance for its Biograph mMR scanner.
In its ruling, the FDA noted that the PET/MRI technology allows the acquisition of images from both modalities simultaneously, without having to move the patient to a different scanner.
"Minimizing changes in a patient's position between tests allows physicians to compare images more easily and helps them get the most accurate information possible," wrote Alberto Gutierrez, PhD, director of the Office of In Vitro Diagnostic Device Evaluation and Safety in the FDA's Center for Devices and Radiological Health (CDRH).
 |
| Siemens demonstrated Biograph mMR at the 2010 RSNA show. |
However, the agency added that people with pacemakers, defibrillators, or other implanted electronic devices should not be scanned with the Biograph mMR system unless those devices are specifically indicated for use in the MRI environment, because the strong magnetic fields of the MRI system may interfere with those devices.
The FDA decision follows last week's news that Siemens received the CE Mark to market Biograph mMR in Europe.
Biograph mMR uses a 3-tesla MRI scanner and PET component inserted into the MRI gantry. The hybrid scanner is installed at a number of sites for clinical research, including University Hospital Klinikum rechts der Isar of the Munich Technical University, Eberhard Karls University Tübingen in Germany, and Massachusetts General Hospital in Boston.
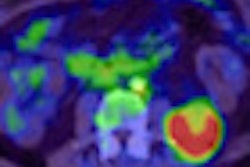
At this week's Society of Nuclear Medicine (SNM) annual meeting, German researchers released a study on the clinical use of PET/MRI, which indicated that the hybrid modality can provide important diagnostic information about soft tissues and physiological functions throughout a patient's body. The technology's ability to find suspicious lesions and potential cancer already appears comparable to that of conventional molecular imaging methods.
The study from TU München evaluated 11 patients with cancer, who underwent single-injection PET/CT followed by PET/MRI with the Biograph mMR. The analysis found that all 13 lesions detected at PET/CT were also identified by PET/MRI, with no significant difference between PET/CT and PET/MRI in uptake ratios.
The study "demonstrates for the first time that newly introduced integrated whole-body MR/PET technology allows simultaneous acquisition of high-quality MR and PET data in a clinical setting within an acceptable time frame," wrote lead study author Dr. Alexander Drzezga.