CHICAGO - Among the highlights in the RSNA 2014 booth of Toshiba America Medical Systems are a new PET/CT scanner, a new interventional x-ray system, a redesigned 3-tesla MRI magnet, and a new upgrade for the company's ultrasound scanner line.
PET/CT
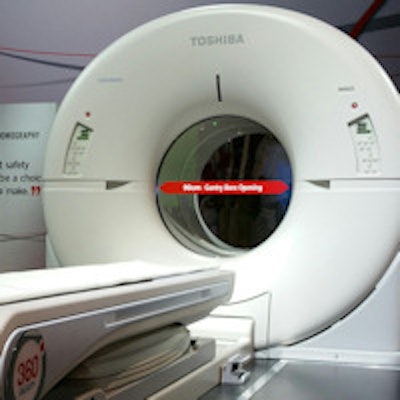
Toshiba is giving RSNA 2014 attendees a first look at Celesteion, a PET/CT scanner first launched at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2014 meeting in St. Louis.
Celesteion was designed with oncology imaging in mind, and as such it is built around Toshiba's Aquilion Large Bore product, with a 90-cm CT gantry for supporting radiation therapy procedures. The scanner is based on time-of-flight (TOF) PET technology, with a resolution of 450 picoseconds and a 70-cm true field-of-view on a 16-detector-row, 32-slice scanner.
 Toshiba's Celesteion PET/CT scanner.
Toshiba's Celesteion PET/CT scanner.Toshiba envisions Celesteion as being appropriate for treatment simulation as well as diagnostic PET/CT procedures, enabling users to make more efficient use of their equipment. The company has received U.S. Food and Drug Administration (FDA) clearance for the system and has sold three systems in the U.S., with the first installation planned for December or January.
The company is also demonstrating new CT detector technology, called PureVision. The technology is built around the more efficient use of x-ray photons, with 40% better light output, meaning that fewer photons are needed to get images of the same quality.
Toshiba developed PureVision through a change to its crystal manufacturing technology that uses microblade cutting to cut detector edges more finely. The detector's digital acquisition system has also been miniaturized to produce less noise.
PureVision will initially be available on the company's flagship Aquilion One scanner, but Toshiba plans to make it available on all new scanners, including Aquilion RXL and Aquilion Prime. The company is expecting FDA clearance for the system around RSNA week.
Finally, Toshiba is rolling out software enhancements across all of its product lines, including AIDR 3D Enhanced, the fourth generation of its adaptive iterative dose reduction (AIDR) technology. AIDR 3D Enhanced produces the same level of dose reduction as the third generation of AIDR 3D, but with improved image quality. It will be rolled out initially for Aquilion One and Aquilion Prime.
A new version 7 software package will include a number of enhancements, including 4D cerebral artery analysis, a new Sure kV dose reduction protocol that enables the scanner to recognize patient size and shape and adjust kV levels accordingly, and organ-effective modulation. AIDR 3D Enhanced is also included in version 7, which is pending FDA clearance and is being shown as a work-in-progress.
Other CT technologies on display include a new power-saving mode that turns off scanner functions if the system sits idle for too long, a work-in-progress cardiac adaptive motion-correction technology for wide-area CT, and 4D airway analysis and 4D cerebral analysis modes.
Interventional x-ray
The big news in the interventional x-ray section of Toshiba's booth is Infinix 4D CT, a combined CT-interventional room designed to address the trend toward interventional rooms with colocated CT scanners.
The concept pairs a ceiling-mounted Infinix Elite C-arm with a Toshiba CT scanner, either an Aquilion Vision or an Aquilion Prime. The setup improves efficiency for users because patients no longer have to be moved from an interventional table to a gurney and through the hospital for a CT scan.
A key point in the configuration is SureGuidance, a Toshiba technique that allows better integration of CT and angiography images by automatically tracking the location where imaging is taking place. Users can note an area on an interventional image, for example, and prompt the CT scanner to go to that exact location.
Toshiba will also promote its Dose Tracking System (DTS) dose reduction software. Toshiba recently received FDA clearance for a new version of DTS designed to be used for neuro procedures. DTS has also received FDA clearance to be used in biplane mode; previously, the software could only be used in single-plane frontal mode.
Ultrasound
A major new upgrade for the Aplio 500, Aplio 300, and Xario 200 scanners, called Platinum, is the highlight in the ultrasound section of Toshiba's booth at this week's meeting.
Platinum is the third and most significant update for the 500 and 300 systems since they were launched in 2011, and it includes enhancements for both diagnostic and interventional ultrasound scanning.
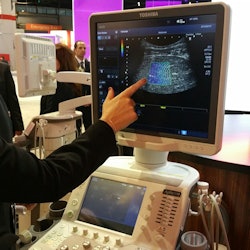 Toshiba's shear-wave elastography provides quantitative analysis and dynamic visual display of tissue stiffness.
Toshiba's shear-wave elastography provides quantitative analysis and dynamic visual display of tissue stiffness.For example, Platinum has extended Toshiba's Superb Micro-Vascular Imaging (SMI) technology for visualizing microvascular flow without contrast to five more transducers, making SMI available on the full range of Toshiba probes, rather than the three it was originally launched with. The new probes with SMI function well with the ability of the Toshiba scanners to penetrate to 40 cm, which can be a boon when examining obese or difficult-to-image patients.
On the interventional radiology side, Toshiba has added Smart Navigation to the Smart Fusion capability it first launched in 2011. Smart Fusion enables users to pull up CT and MRI scans and overlay them on the ultrasound image; Smart Navigation adds a sort of GPS tracking system that provides biopsy guidance, placing a virtual needle tip on the scan that allows users to make sure they are hitting their target, minimizing complications.
Another new interventional technology is Biopsy Enhanced Auto Mode (BEAM), which provides better needle visualization at the push of a button, even if a needle is positioned at a steep angle that is hard to see with traditional ultrasound technology.
All new Aplio 500, Aplio 300, and Xario 200 scanners are now shipping as Platinum systems, and the technology will be offered as a field upgrade.
Beyond Platinum, Toshiba is discussing the addition of shear-wave elastography to Aplio 500, the first time Toshiba has offered the technology. Toshiba has a different take on shear-wave imaging with a technique called propagation mode, which in addition to providing a color map also shows the shear wave as it travels through liver tissue, giving users a method to check the quality of the elastogram. Elastography is currently shipping as part of the Platinum upgrade, but it is only available on Aplio 500.
In new transducers, Toshiba will introduce three new probes. The first is 6CS1, the company's first single-crystal probe. It uses Toshiba's broadband transducer technology, but it also takes advantage of single-crystal design to offer improved penetration. The company believes 6CS1 is well-suited for centers that have a high volume of difficult-to-image patients, or for bariatric centers of excellence.
The other two transducers are replacements for existing Toshiba probes. A new vascular probe, 11L3, replaces an existing transducer with better image quality and a new ergonomic design for vascular work. Toshiba is also rolling out a new curved-volume design for its existing 9CV2 probe that is 50% lighter with better image quality, 4D processing, and volume rendering.
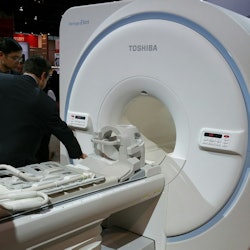 Toshiba is showing its new 1.5-tesla Vantage Elan scanner.
Toshiba is showing its new 1.5-tesla Vantage Elan scanner.Finally, Toshiba has rolled out new designs for its scanner portfolio that make the systems sleeker and more modern-looking.
MRI
In MRI, Toshiba is unveiling a new redesign for its 3-tesla Vantage Titan 3-tesla scanner, first launched in 2009. At the heart of the upgrade are new Saturn gradient options that improve the scanning power of the system, with better image quality and image acquisition.
The company is also showing Vantage Elan, a new 1.5-tesla scanner with a 64-cm bore designed for smaller institutions like community hospitals. Vantage Elan was designed for operation with a low cost of ownership, and it can be sited in a footprint as small as 23 sq meters.
The scanner includes Toshiba's Pianissimo noise-reduction technology, as well as noncontrast protocols for safer exams and an M-Power user interface. The system also includes automated workflow protocols for spine and cardiac exams. First shown at the 2014 European Congress of Radiology (ECR), the scanner has begun shipping in the U.S.