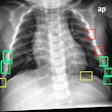
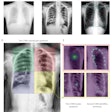
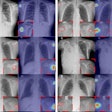
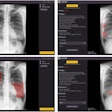
The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to Carestream Health for a bone-suppression software feature for its computed and digital radiography systems.
The optional feature creates a companion image from the original exposure that can improve the visibility of lung nodules and other pathology by suppressing the appearance of ribs and clavicles. The software is available worldwide, according to Carestream.
The current version already offers companion images that can enhance the visibility of tubes, peripherally inserted central catheter (PICC) lines, and pneumothorax, the company said.
In addition, the software's new Integrating the Healthcare Enterprise (IHE) Dose Reporting capability will gather radiation dose information via the IHE profile and direct the information to a PACS.