Siemens Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its new twin robotic x-ray system.
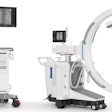
The Multitom Rax (robotic advanced x-ray) is a universal diagnostic imaging system that enables a range of different clinical examinations all in one room using a single x-ray system, according to the company. The twin robotic x-ray system also allows for the acquisition of 3D natural weight-bearing images, which is currently only possible on the system with 3D-capable postprocessing software, Siemens noted.
The system features a height-adjustable patient table and two independent, ceiling-mounted robotic arms for the x-ray tube head and a flat-panel detector. Both robotic arms can be moved into position automatically or manually with motor support to make adjustments. While one robotic arm moves the x-ray tube, the other arm carries the 17 x 17-inch flat-panel detector, which can acquire static, dynamic, and 3D sequences. The operator is always in control of the system's movement and able to position both robotic arms precisely and safely around the patient, according to Siemens.
In cases of a suspected bone fracture, the patient historically has required a CT 3D image to confirm the diagnosis, but with this system, a 3D image can be acquired on Multitom Rax so the patient does not have to wait for a future appointment or be transferred to a CT system.
Built-in features include Comprehensive CARE (combined applications to reduce exposure) and more precise positioning of the system to aid in reducing patient radiation dose, Siemens added.