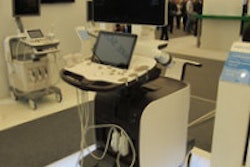
Shimadzu Medical Systems USA has received U.S. Food and Drug Administration (FDA) 510(k) clearance for Trinias, its new family of digital angiography systems.
Trinias is available in three models with Shimadzu's 12-inch Crossover flat-panel digital detectors: a ceiling-mounted system (C12), a floor-mounted system (F12), and a biplane system (B12). The Crossover detector can accommodate any type of angiography study, Shimadzu said. Trinias can also be equipped with an 8-inch flat-panel detector for cardiac procedures as well as neuro exams, Shimadzu said.
All Trinias models feature Shimadzu's Score Pro image processing technology, which performs a real-time evaluation of noise, devices, and anatomy. It then determines the best image quality by optimizing devices and anatomy while eliminating noise, Shimadzu said.
In other Score features included with Trinias, Score RSM provides real-time motion correction during image acquisition, while Score StentView supports percutaneous coronary intervention procedures by displaying stents in a fixed position in real-time.
Score 3D makes use of Shimadzu's C-arm design and 60º per second rotation to acquire 3D images in seconds, according to the firm. Workstation features then allow the user to view images in fly-through or semitransparent modes, or with any angle of images.
Score CT is an application for observing cross-sectional images of low-contrast procedures such as tumor stains, visceral anatomy, and neurointerventional planning, the vendor said. Seven radiation dose reduction features are also included.
Shimadzu showcased the Trinias line as a work-in-progress at the RSNA 2013 meeting in Chicago.