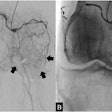
Interventional device developer BioSphere Medical of Rockland, MA, has received Food and Drug Administration clearance for its uterine fibroid embolization (UFE) product, Embosphere Microsphere. The product uses microspheres to block the growth of uterine fibroids by cutting off their blood supply.
Embosphere Microspheres have already been approved for UFE in Europe and Canada. They been approved since April 2000 by the FDA for use in treating hypervascularized tumors and vascular malformations.
By AuntMinnie.com staff writers
November 27, 2002
Related Reading
MRI-guided cryotherapy shows promise as uterine fibroid treatment, July 3, 2002
UFE proves feasible in postmenopausal women, May 13, 2002
BioSphere uterine fibroid embolization device comparable to hysterectomy in study, April 9, 2002
UAE fights fibroids, paves way for pregnancy, April 9, 2002
Pelvic MRI proves more reliable than ultrasound for uterine fibroids, April 8, 2002
Copyright © 2002 AuntMinnie.com