GE Healthcare has long been one of the top two mammography firms, but the company until now hasn't had an offering in the cutting-edge breast tomosynthesis segment. That is changing, however: The firm has begun shipping its SenoClaire tomosynthesis system in Europe and has put the final touches on a regulatory application in the U.S.
Breast tomosynthesis continues to show promise as clinical data accrue demonstrating that the technology reduces recall rates by as much as 30% and finds cancers even in dense tissue -- the Achilles' heel of conventional mammography. Currently, only women's imaging vendor Hologic has U.S. Food and Drug Administration (FDA) clearance for breast tomosynthesis, which it received in 2011 for its Selenia Dimensions system.
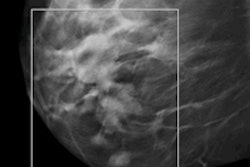
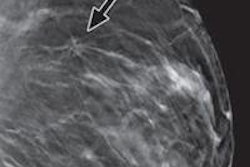
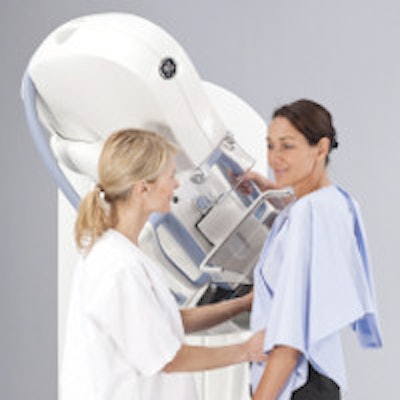
 GE Healthcare's SenoClaire tomosynthesis system. All images courtesy of GE Healthcare.
GE Healthcare's SenoClaire tomosynthesis system. All images courtesy of GE Healthcare.In July, GE received the CE Mark for SenoClaire, and the company has begun shipping the device in Europe, the Middle East, Australia, and Latin America. In the U.S., the vendor submitted the final module of its premarket approval (PMA) application for SenoClaire to the FDA.
Why the PMA, rather than a 510(k) application? It's what the FDA is asking of all companies developing breast tomosynthesis technology, according to Prahlad Singh, general manager for GE's women's health division.
"Even though there is one device on the market, all manufacturers have to go through the same PMA process," he told AuntMinnie.com.
SenoClaire conducts a low-dose short x-ray sweep around the positioned breast, taking nine exposures in a "step-and-shoot" method which removes potential motion from images, because the tube makes a complete stop for each of the exposures. This provides higher peak contrast for microcalcifications than conventional continuous tube motion, according to Singh.
SenoClaire also includes GE's adaptive statistical iterative reconstruction (ASiR) dose reduction technology. ASiR enables the visualization of microcalcifications better than the traditional filtered back-projection algorithms, according to GE.
In addition, SenoClaire's dual-track x-ray tube (made with molybdenum and rhodium) delivers a range of x-ray spectra to penetrate the breast based on breast density and compressed breast thickness, according to GE. The device's automatic optimization of parameters (AOP) technology identifies the densest breast regions and selects the appropriate anode, filter, kV, and mAs to allow for consistent image quality at the most optimal radiation dose.
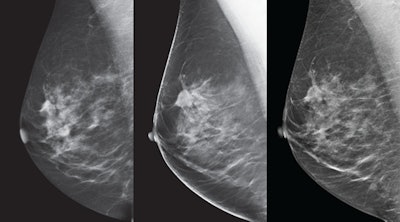 Breast images from the SenoClaire tomosynthesis system. Left: 2D mediolateral oblique (MLO) view. Middle: 3D MLO view. Right: V-preview reconstructed image.
Breast images from the SenoClaire tomosynthesis system. Left: 2D mediolateral oblique (MLO) view. Middle: 3D MLO view. Right: V-preview reconstructed image.And it's dose that counts, GE said: A single mediolateral oblique (MLO) view from SenoClaire is comparable to two-view digital mammography, making the exam half the dose with one compression.
"We pride ourselves on the low dose SenoClaire delivers," Singh said. "From a target dose perspective, SenoClaire's dose is equal to or lower than our competitors'."
GE plans to take advantage of its large installed base to distribute the device, according to Singh, since its Senographe Essential and Senographe Care digital mammography platforms can both be upgraded with SenoClaire.
"All our systems can be upgraded to tomosynthesis -- without a forklift," Singh said.
The company's PMA application includes clinical data that support a SenoClaire function that takes the 3D image and then creates a 2D version, Singh said. But as users are getting comfortable with tomosynthesis, an additional craniocaudal (CC) view may be taken along with the 3D MLO view.
Getting SenoClaire to market is part of GE's overall strategy to offer additional options in women's health, Singh said.
"When we look at breast cancer, we see the whole continuum of care, with mammography as one piece in that puzzle," Singh said. "Our intent is to go to care providers, ask them where the gaps are in their service, and offer a range of technology to address those gaps. SenoClaire is part of that."