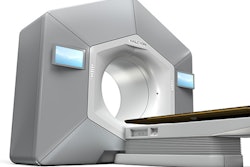
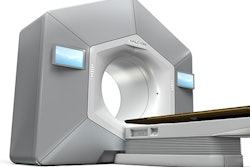
Medical technology company Xcision Medical Systems has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its GammaPod radiotherapy device.
GammaPod targets breast cancer through noninvasive stereotactic delivery of radiation to specific areas of the breast.
The device also minimizes radiation dose to neighboring healthy tissues by immobilizing the breast with a two-layer, vacuum-assisted cup, the company said. GammaPod is not a replacement for whole-breast radiation therapy, the FDA noted.