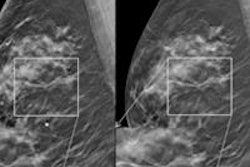
Siemens Healthcare has submitted a premarket approval application (PMA) to the U.S. Food and Drug Administration (FDA) for its Mammomat Inspiration mammography system with a digital breast tomosynthesis (DBT) option.
The company's DBT option has been commercially available and used clinically for diagnosis since 2009 in Europe, Asia, and South America, according to Siemens. But in the U.S., the FDA classifies breast tomosynthesis as class III technology, meaning that data from a clinical trial are needed to measure safety and effectiveness, and the agency requires a PMA submission for clearance, the company said.
Mammomat Inspiration in tomosynthesis mode acquires 25 projection views over a range of 50°, producing 3D DBT images that are intended to be suitable for breast cancer screening and diagnosis.