Philips Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a spectral breast density measurement application for its MicroDose SI full-field digital mammography (FFDM) system.
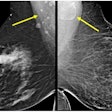
According to the company, it is the first clearance of a spectral breast density measurement tool, through which adipose (fat) and glandular tissue can be differentiated to measure volumetric breast density.
The application measures the glandularity and thickness in each pixel of an image to calculate the total volume and volumetric percentage of glandular tissue in the breast, Philips said.
Once the calculations are completed, the examination is assigned a MicroDose density score that correlates with BI-RADS. The measurement is displayed on the review workstation in the DICOM tag of the acquired image and is exported for display in a DICOM structured report.