Breast cancer patients with cardiac pacemakers implanted on the same side of the body as the diseased breast may require surgical relocation of the device prior to radiotherapy. However, if the patient qualifies for accelerated partial-breast irradiation (APBI) using high-dose brachytherapy, the surgery might not be necessary.
Dayee Jacob, senior medical physicist at Christiana Care Health System, described how he prepared a treatment plan for the first two patients with pacemakers at Christiana's Helen F. Graham Cancer Center who were candidates for breast conservation surgery and APBI treatment. By coincidence, one had a left-side pacemaker and required treatment for the left breast, and the other had a right-side pacemaker and needed treatment for the right breast.
Jacob delivered a poster presentation at the American Association of Physicists in Medicine (AAPM) annual meeting held midsummer in Charlotte, NC. He subsequently discussed the treatment plan in greater detail with AuntMinnie.com.
The concern about treating a patient with an implanted pacemaker is that direct or scatter radiation can cause partial or complete malfunction of the device, putting the heart at risk. In 1994, AAPM recommended a maximum accumulated dose limit of 2 Gy for pacemakers.
As a result, regardless of the type of radiotherapy for breast cancer, if a pacemaker is on the same side as the treatment, it is typically relocated to the other side of the body in a separate surgical procedure.
Jacob and colleagues believed that they could develop a treatment plan for their two patients that would deliver the prescribed radiation dose without compromising the pacemakers' functionality. The plans were developed, but, ultimately, one was not used because the patient had positive margins following a lumpectomy (the patient underwent a mastectomy). The other patient, a woman in her 70s with cancer in her right breast and an in situ pacemaker (Model ADDR01, Medtronic), successfully completed the treatment.
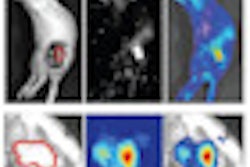
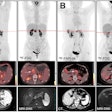
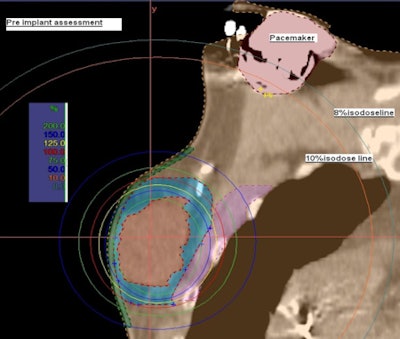 |
| Preimplant assessment. All images courtesy of Dayee Jacob. |
Prior to breast conservation surgery, the patient had a CT scan so Jacob could determine how far the pacemaker was located from the cancerous tissue. He performed a simulation to create a virtual catheter inside the reconstructed image to estimate the radiation dose that brachytherapy would deliver to the pacemaker. The initial simulation and preliminary estimate showed that the pacemaker dose would be about 8% of the prescribed dose, exceeding 2 Gy.
However, a simulation using a seven-catheter strut-adjusted volume implant (SAVI) (Cianna Medical) produced an acceptable result. It showed that the dose could be reduced to 130 cGy to 140 cGy, and that the dose to the lung, chest wall, and skin surface also would not exceed acceptable levels.
After surgery in which the SAVI device was implanted in an optimal direction for brachytherapy treatment, the patient had another CT scan of the implanted breast with images acquired in 2-mm-thick slices, which is required for treatment planning.
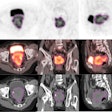
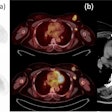
The SAVI device, which when extended looks like a small eggbeater in the breast, encompassed the lumpectomy cavity and has radio-opaque markers on the catheters. A 3D treatment planning system (Oncentra Brachy, Nucletron) with multiplanar reconstruction allowed Jacob to three-dimensionally reconstruct the device, showing exactly how it sits in the patient, he explained.
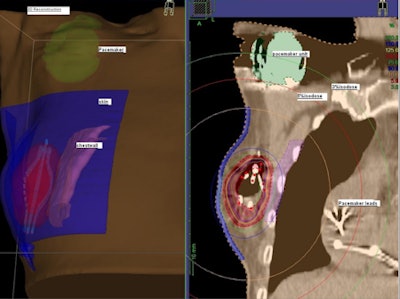 |
| A 3D reconstructed view of the implanted SAVI device. |
Jacob then performed contouring and defined the constraints using the software. With the dose optimization tools of the planning system and user-defined criteria, the software determines source dwell time for multiple dwell positions of each catheter. Further modifications to the dose distribution are done manually, if necessary, to meet the dose tolerance criteria of the at-risk organs -- namely, the chest wall, ribs, lung, skin, and pacemaker structure.
For this patient, the target volumes (V100, V95, and V90) were 90.1%, 95.5%, and 98.5%, respectively. The pacemaker was exposed to calculated average point doses of 140 cGy.
As a standard quality assurance measure, computer-generated treatment plans for any patient are verified using another independent software program. On average, the entire process of treatment planning -- from acquisition of the CT images at the treatment planning workstation through pretreatment quality assurance -- takes about two hours.
Prior to and following the treatment, a representative from the pacemaker manufacturer checked the parameters of the pacemaker, providing a copy of the findings each time to Jacob. Both evaluations produced normal results.
"We believe that we've shown that iridium-192 high-dose-rate brachytherapy treatment can be safely delivered for breast cancer patients with an implanted pacemaker on the same side of the treatment," he concluded. "We hope that our experience convinces other cancer centers providing this treatment to offer it to these patients."