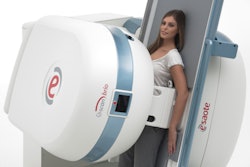
The U.S. Food and Drug Administration (FDA) has cleared ultrasound vendor Esaote North America's MyLab Gamma ultrasound scanner.
The portable unit is laptop-sized and can be used in a variety of clinical environments, according to the firm. It is ergonomically designed and can operate on battery power, and its two probe connectors can be expanded to four with an optional cart.