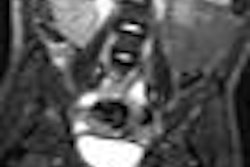
Focused-ultrasound developer InSightec has received U.S. Food and Drug Administration (FDA) approval to launch a phase III trial of its ExAblate Neuro system for the treatment of essential tremor.
ExAblate Neuro uses MR-guided focused ultrasound therapy to provide an incision-less treatment through the intact skull, with no ionizing radiation, the company said.
The multicenter, double-blind, controlled trial will include a one-year follow-up period. Patients in the trial will be randomized to receive either ExAblate treatment or no treatment. Enrollment is expected to begin in mid-2013, according to the vendor.