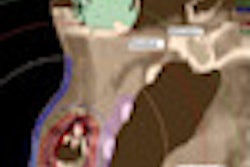
Radiation oncology firm Elekta has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Clarity 4D monitoring software, which monitors motion in the prostate and surrounding tissues and organs during radiation therapy for prostate cancer.
The software continuously visualizes the prostate's location in real-time and with submillimeter accuracy during the delivery of radiation, according to the company. It is designed to enable hypofractionation techniques that shorten the treatment schedule.