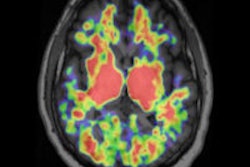
CHICAGO - New technologies being launched by Philips Healthcare at this week's RSNA meeting include a PET/CT scanner with new digital detectors, a CT system optimized for spectral imaging, and an ultrasound platform designed for both cardiology and radiology applications.
Molecular imaging
The new PET/CT scanner is called Vereos, and it's been designed for full digitization of photon signals, with Philips replacing the photomultiplier tubes (PMTs) previously used in conventional PET cameras with digital detectors.
The scanner uses what Philips calls digital photon-counting technology: Rather than feeding signals from scintillation crystals to PMTs that each gather data from several crystals, each detector is coupled with a scintillation crystal on a one-to-one basis, which improves the system's linear response and thus image quality, according to the company. Philips claims the system has twice the volumetric resolution, sensitivity, and quantitative accuracy as other systems.
 Philips' new Vereos PET/CT scanner.
Philips' new Vereos PET/CT scanner.The PET and CT modules are now housed in a single gantry rather than a double gantry, and Vereos also uses other Philips technologies found on its previous PET/CT systems, such as time-of-flight (TOF) PET, iDose4 radiation dose reduction, and iPatient workflow.
Vereos has received 510(k) clearance from the U.S. Food and Drug Administration (FDA), and Philips plans to begin shipping in 2014.
For the Ingenuity TF PET/CT scanner launched at the 2012 RSNA meeting, Philips is reporting that dozens of systems have been sold globally. Features on the system include the Astonish TF PET algorithm and iDose4 CT dose reduction features, as well as the company's metal artifact reduction for large orthopedic implants (O-MAR) technique.
The company is also promoting how technological improvements pioneered in its CT division are disseminated quickly to the hybrid scanner. The Ingenuity TF Elite package makes available the new NanoPanel Elite CT detector design, which produces a more pure electronic signal that allows low-energy x-ray techniques without distortion.
Another feature of the package, iPatient, gives radiologists workflow enhancement tools that enable them to create more consistent imaging protocols, while SyncRight improves communication between the CT scanner and contrast injection system.
PET/MRI is a focus in Philips' booth, with the company discussing the capabilities of its Ingenuity TF system, which is based on PET and 3-tesla MRI scanners in tandem mode, with a table that shuttles patients between each scanner. Philips believes the configuration enables higher performance than systems in which a PET detector ring is located inside the MRI gantry.
On the SPECT side, Philips is highlighting the BrightView XCT gamma camera, in particular its full iterative technology (FIT) feature for better image quality. Concurrent imaging enables multiple image acquisitions without longer exam time, and the company believes that the system's co-planar SPECT/CT technique improves accuracy in cardiac attenuation correction.
Finally, Philips has added new molecular imaging applications to its IntelliSpace Portal client-server processing and review workstation, including tools for collaboration with referring physicians. The company is also in the process of adding applications for viewing images acquired with new amyloid PET tracers that are now commercially available.
CT
The new Philips CT scanner is called IQon Spectral CT, and it represents a new approach to spectral imaging, in which signals from two x-ray energies are analyzed to determine changes in tissue composition.
Philips designed the system with two parallel sets of 4-cm-wide detectors that are capable of distinguishing between x-ray photons of high and low energies simultaneously, and scans are reconstructed in colors that represent the different compositions of tissue and organs in the body. Philips believes its detector-based approach is superior to other techniques based on CT x-ray sources for dual-energy imaging.
After a spectral CT exam, radiologists might review the conventional grayscale image, then consult with the color spectral CT data for additional information that was acquired with the same scan. Spectral CT could be used for all routine imaging, according to Philips.
The spectral CT capabilities for the IQon scanner are pending 510(k) clearance, and the scanner is running at several sites on an investigational basis. Philips sees the system as a premium unit that will not replace the other scanners in the company's product line.
 The new IQon Spectral CT scanner.
The new IQon Spectral CT scanner.Philips is also highlighting technologies shown at previous RSNA meetings, such as its iterative model reconstruction (IMR), the newest generation of the company's iterative reconstruction technology for reducing dose in CT scanning.
First shown at RSNA 2011, IMR reduces dose by 60% to 80%, with improved image quality compared to filtered back projection. Most reconstructions can be performed in fewer than three minutes, making IMR suitable for routine use in the CT department for a variety of clinical applications.
As mentioned, another highlight this year is iPatient, which is designed to improve operator-to-operator consistency in CT scanning. First shown at the 2012 RSNA meeting, iPatient began shipping in 2013.
Available for the iCT and Ingenuity CT scanners, iPatient enables users to plan results, not acquisitions, according to Philips, and includes patient-specific optimization of image quality and radiation dose. Meanwhile, SyncRight enables users to determine the appropriate contrast dose for a scan while maintaining consistent image quality.
Philips is also highlighting its NanoPanel Elite detectors, which began shipping in the second quarter of 2013. The technology simplifies the design of the detector modules, and enables improved image quality at low x-ray energies and currents.
Ultrasound
The new Epiq ultrasound platform is the big highlight in the ultrasound section of Philips' booth. First launched in Europe in September 2013 at the European Society of Cardiology Congress, RSNA 2013 marks the radiology debut for the technology.
Epiq was eight years in the making and represents Philips' flagship ultrasound technology, replacing the iU22 scanner. It is designed to serve a wide range of clinical environments, including radiology, cardiology, and ob/gyn.
Epiq technology is based on a new precision beamformer with massive parallel imaging, which improves speed and image quality. It also combines the company's xMatrix transducer technology with real-time 3D, producing high temporal resolution and uniformity even in difficult-to-image patients.
Philips believes the Epiq design overcomes barriers that traditional ultrasound faces in imaging obese patients, for example; this could reduce the referral of patients to CT and MRI because ultrasound can't be used. Epiq's new transducers have up to a 76% increase in penetration with more uniform imaging: The system has imaged patients with a body mass index (BMI) up to 80 with high image quality, according to the company.
In addition, Epiq is capable of acquiring panoramic images with a full view of organs, which enables radiologists to have a richer dialog with referring physicians. It has also been integrated with Philips' PercuNav ultrasound-guided needle tracking system for interventional procedures.
Philips also designed the Epiq platform with an eye toward improved workflow, specifically to minimize repetitive stress injuries among sonographers. The system is 40% lighter, with less physical reach required to access buttons and fewer steps needed to get an image. The company has also built technology into Epiq to improve the reproducibility of images, which Philips believes will result in a 30% reduction of repeat exams.
On the image management side, images acquired with Epiq can be viewed side by side with MRI and CT studies on the company's IntelliSpace Portal workstation.
Philips has received FDA clearance for Epiq and has begun shipping.
Interventional
AlluraClarity is the big highlight in the interventional imaging section of Philips' RSNA booth. Cleared by the FDA in 2013, AlluraClarity is the company's next-generation angiography platform.
The system includes ClarityIQ, a hardware and software technology for producing high levels of image quality at ultralow radiation doses, according to Philips. ClarityIQ also includes features for motion compensation and noise reduction.
Philips is showing panels in its booth that demonstrate the lower radiation doses possible, compared with its older Allura Xper system, for applications such as cerebral digital subtraction angiography (DSA) and cerebral fluoroscopy. A tool called pixel shift removes image artifacts and smoothes out background information, while an image enhancement tool enables physicians to customize images in a manner similar to a stereo equalizer.
New 3D tools available with AlluraClarity include XperCT release 3, with metal artifact reduction, ultrafast data reconstruction, and filtering based on patient BMI, while XperGuide Ablation includes enhanced planning and feedback for ablation procedures, and viewing of the isotherm for needles.
Women's imaging
At this year's RSNA show, Philips is highlighting its next-generation MicroDose unit with single-shot spectral imaging (SI) technology, together with its first clinical application, the work-in-progress Spectral Breast Density Measurement package.
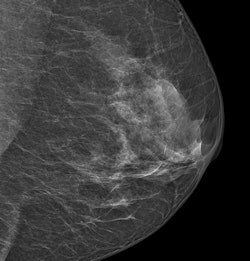 An image acquired with Philips' MicroDose SI system. Image courtesy of Philips.
An image acquired with Philips' MicroDose SI system. Image courtesy of Philips.MicroDose SI uses digital photon-counting technology, which allows clinicians to conduct exams using low radiation dose without compromising image quality; the company received 510(k) clearance from the FDA for the device in February.
High breast density is a known risk factor for breast cancer, and interpretation of mammograms of dense breast tissue is more difficult. Philips' breast density software is the first application the company has developed to interpret the spectral data obtained from MicroDose SI by giving radiologists a density score that works with BI-RADS classifications, according to the company.
Philips is also highlighting its IntelliSpace Breast workstation, a multimodality workstation that integrates mammography, ultrasound, and MRI studies so that clinicians get a comprehensive picture of multiple breast exams from a single workspace. IntelliSpace Breast also incorporates BI-RADS standard reporting for mammography, ultrasound, and MRI to provide consistency.
PACS
On the PACS side, Philips is highlighting IntelliSpace Portal, an add-on to its IntelliSpace PACS that allows users to view and process exams from CT, MR, and ultrasound scanners and perform advanced visualization studies on a PACS workstation.
New for IntelliSpace Portal this year is ultrasound viewing (with a multimodality viewer) and a set of advanced viewing tools, including cardiac MR, neuro MR, and work-in-progress CT transcatheter aortic valve implantation (TAVI) planning.
Also new this year for IntelliSpace Portal is Web Collaborator, which allows radiologists and referring physicians to share images and discuss diagnosis and treatment decisions.
X-ray
In mobile x-ray, Philips is demonstrating SkyFlow, a work-in-progress application for its Mobile Diagnost wDR unit that offers the same image contrast without a grid as with a grid. The company plans to file for 510(k) clearance for the technique soon.
Philips is also showcasing classic radiography systems in its booth, such as the DuraDiagnost economy-level unit being show as a work-in-progress. The unit is shipping globally in a variety of configurations, from two fixed detectors to a single wireless detector.
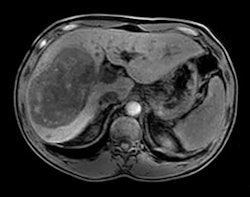 An mDixon scan using Philips' Ingenia MRI system with dStream. Image courtesy of Philips.
An mDixon scan using Philips' Ingenia MRI system with dStream. Image courtesy of Philips.DuraDiagnost is a floor-mounted system that saves on the siting costs that might be involved with a ceiling-mounted unit, and it also sports manual rather than motorized controls. However, the system has the same digital detectors and Eleva workstation user interface found on more advanced Philips radiography systems.
MRI
Upgrades and enhancements are being highlighted in the MRI section of Philips' booth, such as SmartPath to dStream, the company's digital broadband MRI technology. The firm is touting the latest release of dStream, which includes applications in neurology, trauma, pediatrics, and fatty liver patients, as well as routine fat saturation.