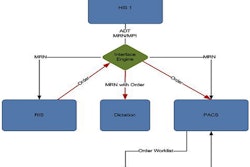
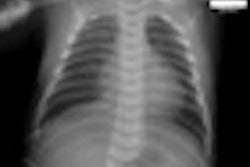
Radiology informatics firm Aycan Medical Systems has received 510(k) clearance for its Apple iPad app for teleradiology from the U.S. Food and Drug Administration (FDA).
The aycan mobile software program provides wireless, portable access to medical images and should be used only when there is no access to a diagnostic workstation. It is intended for use only to display medical images for diagnosis from CT and MRI exams.
The teleradiology app previously received CE Mark approval for use in Europe.