Say the words "gadolinium-enhanced MRI" and "renal imaging" in the same breath-hold, and three little letters will likely leap to mind: NSF. Nephrogenic systemic fibrosis continues to puzzle (some might say plague) the MR imaging community, with researchers speculating on its cause while the number of reported cases continues to grow.
As of spring 2007, 215 cases had been reported to the NSF Registry. According to the U.S. Food and Drug Administration (FDA), 250 cases had been reported to its MedWatch list as of September 2007. Possible theories behind the NSF-gadolinium connection have included contrast agent stability, the risk of renal failure, and the upregulation of transglutaminases.
NSF aside, patients with renal disorders must still undergo imaging exams. MRI remains the best option for detecting and grading kidney disease. Two recent studies in Radiology and the American Journal of Roentgenology offered advanced techniques for performing MR angiography (MRA) and MRI in the renal arteries. In both cases, gadolinium-based agents were used successfully and without any serious side effects.
It's important to note that these research projects were not specifically designed or intended to address NSF. However, they do offer techniques -- lower dose of contrast agent, accelerated imaging -- that could benefit patients with a range of renal disorders. In addition, the study authors updated AuntMinnie.com on how the NSF situation has affected how renal MRI is performed at their respective institutions.
Extracting RBF from perfusion MRI
Phase-contrast imaging is the current standard for measuring renal blood flow (RBF), but it must be performed separately for each kidney. In addition, phase-contrast imaging must be done orthogonal to the vascular vector flow, which can lead to technical difficulties in angled and tortuous arteries. Instead, Dr. Diego Martin, Ph.D., and colleagues outlined a perfusion method for RBF measurement as an alternative to phase-contrast imaging.
Martin's group is from Atlanta's Emory University School of Medicine and the Children's Hospital of Atlanta. For this research, 29 participants with normal renal arterial anatomy were enrolled. Based on angiography results, these subjects also had renal arteries that were free of stenotic lesions. Among the 29 volunteers, nine had no history of renal disease or hypertension while another dozen had either renal hypertension or ureteropelvic junction obstruction. Overall, 56 kidneys were analyzed (27 left, 29 right).
Imaging was done on a 1.5-tesla scanner (Intera, Philips Healthcare, Andover, MA). Seven gadolinium chelate-doped plasma phantoms were used to assess the behavior of MR signal intensity (SI). Gadopentetate dimeglumine (Magnevist, Bayer Schering Pharma, Berlin) concentrations varying from 0 mmol/L to 30 mmol/L were used. The number of dynamic images was set at 10, and SI measurements were performed from the fifth to the 10th dynamic image.
In the subjects, single-kidney RBF phase-contrast imaging was done in the transverse and coronal planes using a steady-state balanced fast field-echo technique. For single-kidney RBF gadolinium-enhanced renal perfusion imaging, 0.1 mmol/kg of gadolinium chelate was diluted in 60 mL of normal saline with imaging initiation five seconds after administration. Imaging was performed during normal breathing and during the first pass using the T1 high-resolution isotropic volume examination (THRIVE) technique. Motion due to respiration was corrected manually.
For image analysis, each total perfused kidney volume was segmented using a semiautomated algorithm based on SI thresholds set by co-authors John Votaw, Ph.D.; Puneet Sharma, Ph.D.; and Dr. Khalil Salman. The analysis also included a dynamic SI mask in the descending aorta to serve as the input function. The Kety-Schmidt integral was used to model the uptake of gadolinium chelate in the kidneys. Finally, interobserver variation was done by Martin, Sharma, Salman, and co-author Dr. Thomas Lauenstein.
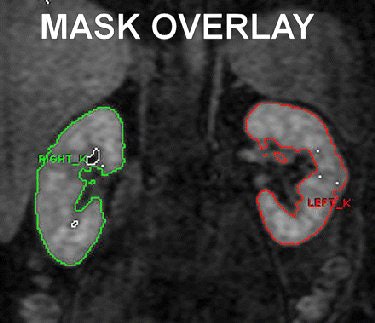 |
| Shows coronal MR image of dynamic 3D gradient-echo RBF perfusion sequence (3.7/1.7; field-of-view, 430 mm² matrix, 96 [reconstructed to 256]; flip angle, 30°; sections, 30; and section thickness, 2.8 mm). ROI tracing for each kidney represents volumetric binary kidney mask created semiautomatically by means of threshold and erosion/dilation steps. Overlay shows final analytic step before mask is propagated through appropriate dynamic series while manually correcting for kidney motion; this process is repeated for each section. Software is configured to sum number of voxels and SI in mask, which can subsequently help determine kidney volume and gadolinium-chelate perfusion (Equation). Similar mask (not shown) was used to help determine aortic SI. Fig 1. Martin DR, Sharma P, Salman K, et al. "Individual Kidney Blood Flow Measured With Contrast-enhanced First-Pass Perfusion MR Imaging." Radiology 2008; 246:241-248. |
In the phantoms, the group found that the MR SI rose linearly until reaching approximately 3.5 mmol/L gadolinium chelate concentration, after which the curve demonstrated nonlinear traits.
In the volunteers, the 3D perfusion images were acquired without complication. When comparing the latter images to phase-contrast imaging, the authors found that the average difference between both measurement techniques for all 56 kidneys was 0.08 mL/sec ± 1.8 (standard deviation). In the left kidney, the average of differences between RBF and phase-contrast imaging (true mean bias) was 0.32 mL/sec (95% confidence interval [CI]). For the right kidney, the true mean bias was -0.18 mL/sec (95% CI).
"The measurement of RBF by using first-pass perfusion showed interobserver reproducibility is on average within 10% of the average measured flow, which we consider to be an acceptable degree of precision," Martin's group wrote. "Such performance may be adequate for certain clinical investigations (such as) left-right perfusion comparisons ... or longitudinal evaluation to assess progress or improvement of disease" (Radiology, January 2008, Vol. 246:1, pp. 241-246).
The group noted that RBF estimation using this method does require that the total amount of gadolinium chelate in the kidney, as well as its concentration in the artery, be determined. "By making sure that the kidney (region of interest [ROI]) is at least as large as the perfused portion of the kidney and that the artery ROI is smaller than the artery, the analysis is relatively insensitive to ROI placement," the authors explained.
Subjects with renal insufficiency were not part of this study because of limitations set by the Institutional Review Board, which precluded the enrollment of patients with impaired renal function, Martin told AuntMinnie.com.
"Our current understanding of NSF shows ... patients with even severe stage 5 renal dysfunction have an immeasurably small risk of NSF when using gadolinium chelates with higher conditional stability, such as cyclic agents," Martin wrote in an e-mail. "For the past year, we have converted to such agents, and now use ProHance (gadoteridol, Bracco Diagnostics, Princeton, NJ) for renal functional studies. We limit cumulative dose, in patients receiving multiple studies, to 0.3 mmol/kg lifetime dose."
Martin added that he recently completed a review of other potential risk factors for NSF in addition to renal insufficiency.
Lower dose, higher field strength
The second study was led by Drs. Christoph Herborn, Val Runge, and colleagues at the Scott and White Clinic and Hospital at Texas A&M University Health Science Center in Temple. Herborn is now at the Medical Prevention Center Hamburg (MPCH) at Medical University Center Hamburg-Eppendorf in Germany.
The group performed an intraindividual comparison between double-dose contrast at 1.5-tesla MRA and standard dose 3-tesla MRA.
For this prospective study, 21 patients with hypertension of unknown origin were enrolled. They had been referred for the diagnosis or exclusion of renal arterial stenosis. The mean serum creatinine concentration in this group was 1.4 ± 0.5 mg/dL, and the mean serum urea concentration was 45.2 ± 22.7 mg/dL.
Imaging was done on a 1.5-tesla (Magentom Sonata, Siemens Medical Solutions, Malvern, PA) and a 3-tesla scanner (Trio, Siemens). For the 1.5-tesla exam, gadodiamide was administered (Omniscan, GE Healthcare, Chalfont St. Giles, U.K.) at a dose of 0.2 mmol/kg. For the 3-tesla studies, the dose was 0.1 mmol/kg. The MR sequences included coronal 2D steady-state precession, for localizing renal artery origin, and coronal breath-hold fat-saturated spoiled gradient-recalled echo imaging.
Two radiologists, blinded to the dose of contrast material, assessed the imaging data (side by side) in consensus for renal artery disease and image quality on a four-point Likert-type scale with 1 representing nondiagnostic and 4 excellent visualization.
According to the results, 45 renal arteries were detected in 21 patients, and five renal arterial stenoses were seen equally well with both magnets. Of the 21 patients, three had supernumerary renal arteries and none was missed on the 3-tesla datasets. The overall sensitivity and specificity of 3-tesla MRA for detecting relevant luminal changes in the renal arteries were both 100%, the authors reported.
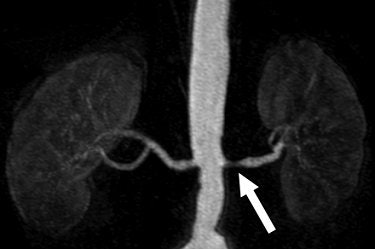 |
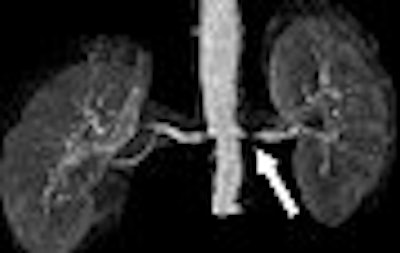
| A 68-year-old man with severe stenosis of left renal artery. Anteroposterior maximum intensity projections of coronal 3D MR angiograms of abdominal aorta and renal arteries obtained with 0.2 mmol/kg of gadodiamide at 1.5 tesla (above) and 0.1 mmol/kg of gadodiamide at 3 tesla (below) show stenosis (arrow) equally well. |
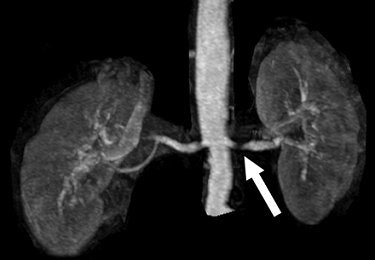 |
| Herborn CJ, Runge VM, Watkins DM, Gendron JM, Naul JG, "MR Angiography of the Renal Arteries: Intraindividual Comparison of Double-Dose Contrast Enhancement at 1.5 T with Standard Dose at 3 T" (AJR 2008; 190:173-177). |
However, 3-tesla MRA with the lower dose did not perform as well as double-dose 1.5-tesla MRA for visualizing the maximal vessel length -- 35 mm on 3-tesla MRA versus 48 mm on 1.5-tesla for the right renal artery, and 32 mm versus 42 mm for the left renal artery.
In addition, the mean signal-to-noise ratio (SNR) of the renal arteries on double-dose 1.5-tesla images came in at 125.7 ± 12 versus 112.3 ± 11 on single-dose 3-tesla images. The same held true for the mean contrast-to-noise ratio (CNR), which was higher on the 1.5-tesla images (35 ± 9) than on 3-tesla ones (23 ± 13). Finally, intraparenchymal branches were more visible on 1.5-tesla imaging.
In terms of the image quality score assigned by the readers, 3-tesla MRA came in at 3.5 ± 0.4 while 1.5-tesla came in at 1.6 ± 0.3, a difference that was not statistically significant, the authors stated.
"Our findings emphasize that double doses are not needed at the higher field strength to acquire renal MR angiographic datasets of sufficient diagnostic quality," they concluded. "It is likely that improvements in technique, particularly parallel imaging and time-resolved 3D MR angiography, will further improve contrast-enhanced renal MRI angiography at 3 (tesla)." Other techniques that show promise for increasing spatial resolution are spatial harmonics and sensitivity encoding, the group added (AJR, January 2008, Vol. 190: 1, pp. 173-177).
Runge and Herborn took the time to answer some questions about the ever-shifting role of gadolinium-enhanced MRA in this particularly tricky patient population.
AuntMinnie: Is Omniscan still used at your institution (Scott and White and/or MPCH), or has there been a change to another gadolinium-based agent?
Runge: Omniscan is no longer used at Scott and White. Its use was discontinued very soon after the initial FDA advisory on June 8, 2006. We switched to the only macrocyclic chelate (ProHance) currently available in the U.S. (because) this class of agent (is) substantially more stable in vivo than Omniscan.
(ProHance) has been unfortunately "labeled" by its competitors since its launch as having more "side effects." This is not true as attested (to) by the 10 technologists at our site who have used this agent -- and only this agent -- for the past year and a half, and who've seen no difference in side effects relative to the former use of Omniscan. This includes two technologists who were thoroughly convinced that (ProHance had) more "side effects" prior to our switch.
It was important, however, to provide appropriate information regarding this agent and the reason for the switch, when this was done, as all those in the health profession have preconceived biases, which are often inadvertently transmitted to the patient.
Herborn: We still use Omniscan, along with other marketed agents. Omniscan has the advantage of being an agent well-proven for all indications throughout the body and the brain. Since we are performing whole-body MRI examinations in the vast majority of cases at MPCH, we need an agent which combines both vascular and parenchymal contrast. ProHance is also used at MPCH (and), unfortunately, tends to cause mild side effects (especially nausea) more frequently. Therefore, we are also injecting other compounds.
However, Omniscan has been contraindicated in patients with renal failure (i.e. GFR < 30 mL/min/1.72m²) and liver transplantation in Europe since February 2007. In spite of this, such patients are hardly ever seen at our institution.
Do you perform eGFR prescreening in these patients before moving ahead with the MRA exam?
Herborn: All patients at our institution have onsite lab testing of their serum creatinine levels and the eGFR prior to contrast administration since spring 2006. In case of renal insufficiency, we mostly get along without any dye and only inject lower doses if truly mandatory. We have not seen any case of NSF among our patients at MPCH.
Runge: We now prescreen patients with eGFR, and then make a medical decision as to whether to proceed with contrast injection. This is mandated by the FDA. However, there are only rare instances when the decision is made not to give contrast, since there is only one known possible NSF case with ProHance, and that an extremely mild case (two isolated skin patches, with no other findings), which occurred following six double doses. However, we do not use above 40 mL for any exam.
Anecdotally, did you receive feedback from your nephrology colleagues about the 3-tesla images?
Herborn: No, these guys do not really see a difference between 1.5-tesla and 3-tesla. However, they care about NSF and the dose of gadolinium injected in their patients, which might be reduced at the higher field strength.
There is currently a debate over noncontrast MR versus more refined contrast-enhanced MR techniques in patients with renal insufficiency. Which avenue you think is more worthwhile to pursue?
Herborn: There is definitely such a debate -- Dr. Runge and I also published a study on noncontrast-material SSFP (steady-state free precession) MRA of the renal arteries, which is an alternative in these patients. SSFP, as well as fresh-blood MRA or other techniques (e.g., phase-contrast MRA) are re-evaluated in view of NSF (Radiology, April 2006, Vol. 239:1, pp. 263-268).
This is a growing concern among radiologists and will surely play a more important role in the near future.
Runge: The University of California, Los Angeles (UCLA) group is one of the leading groups in the application of parallel imaging for improvements in contrast-enhanced MRA (Investigative Radiology, June 2007, Vol. 42:6, pp. 392-398, 477-483).
As an additional point, new dynamic and almost time-resolved contrast-enhanced MRA sequences, such as TWIST (time-resolved angiography with interleaved stochastic trajectories) and TREAT (time-resolved echo-shared angiography technique), are likely to lead to lower dose of gadolinium chelate, an important issue for NSF.
By Shalmali Pal
AuntMinnie.com staff writer
January 10, 2008
Related Reading
GFR no better than creatinine for predicting renal failure, December 18, 2007
MR experts take issue with FDA gadolinium warning, September 18, 2007
Gadolinium: A 'necessary factor' in the development of NSF? March 27, 2007
Noncontrast MR: The solution to the NSF problem? July 26, 2007
Copyright © 2008 AuntMinnie.com