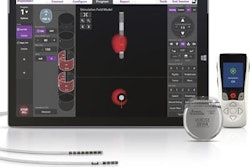
Interventional and ultrasound technology developer Boston Scientific has received clearance from the U.S. Food and Drug Administration (FDA) for its Precision Montage MRI spinal cord stimulator system.
Precision Montage is designed to allow patients to receive full-body 1.5-tesla MRI scans, along with the company's MultiWave technology. MultiWave enables the delivery of multiple waveforms to relieve chronic back pain, Boston Scientific said.