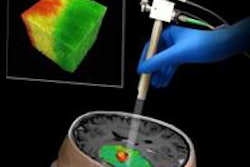
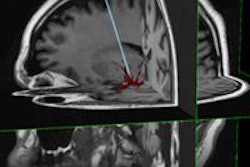
The U.S. Food and Drug Administration (FDA) has cleared Deerfield Imaging's Symbis stereotactic brain biopsy surgical system.
Deerfield was previously known as IMRIS. IMRIS submitted the 510(k) application to the FDA late last year, the company said.