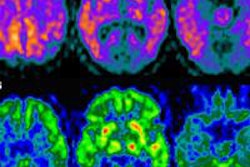
GE Healthcare has filed for 510(k) clearance of its new whole-body Signa PET/MR scanner with the U.S. Food and Drug Administration (FDA).
The unit combines simultaneous image acquisition from GE's 3-tesla MR technology and PET, and it is designed to help physicians improve scan efficiency, particularly in oncology, neurology, and cardiology applications, GE said.
Signa PET/MR features GE's MR-compatible silicon photomultiplier detector (SiPM) technology, a digital detector that is up to three times more sensitive than conventional PET technology, according to the company. It also offers fast coincidence timing resolution to enable time-of-flight (TOF) reconstruction.
The Signa PET/MR system is fully upgradeable from GE's Discovery MR750w 3-tesla MRI unit, GE added.
GE has installed research systems at Stanford University; the University of California, San Francisco; and the University of Zurich.