A new study by Japanese researchers published on May 5 in Radiology confirms previous findings that traces of gadolinium contrast remain in the brains of people who receive contrast-enhanced MRI scans. The results are raising new questions about the safety of gadolinium-based contrast, such as whether the deposits are harmful.
The study comes from researchers at Teikyo University in Tokyo, who found traces of gadolinium in autopsy specimens of five people. The subjects had significantly higher levels of gadolinium in the dentate nucleus and globus pallidus regions of the brain than five people who never received gadolinium-based contrast agents (GBCAs) (Radiology, May 5, 2015).
Because lead author Dr. Tomonori Kanda, PhD, and colleagues did not include histopathologies of the brains as part of the study, they could not determine if there was any toxicity from the residual gadolinium and what effect it might have had on the brain.
"The first question has to be: Is it harmful?" said Dr. Emanuel Kanal, director of MR services and professor of radiology and neuroradiology at University of Pittsburgh Medical Center (UPMC), to AuntMinnie.com. "Until recently, we didn't even know it existed. It is very fair to say no one has shown it to be harmful, but since we didn't know it existed, no one has ever studied this. We just don't know."
 Dr. Emanuel Kanal, director of MR services at UPMC.
Dr. Emanuel Kanal, director of MR services at UPMC.Kanal was not involved in the Japanese study, but he co-authored an online editorial with Michael Tweedle, PhD, from Ohio State University on the potential clinical implications of gadolinium traces in the brain. The editorial was also published in Radiology.
Gadolinium's presence
On its own, gadolinium can be toxic. When used in contrast agents, gadolinium bonds with a molecule called a chelating agent, which controls the distribution of gadolinium within the body.
Safety concerns about gadolinium erupted in 2006, when the use of GBCAs was linked to the development of nephrogenic systemic fibrosis (NSF), a debilitating and sometimes fatal condition. The incidence of NSF was cut dramatically, however, with the development of clinical protocols that restrict the use of gadolinium contrast in patients with impaired renal function.
But recent studies have raised new concerns about gadolinium's safety, particularly regarding whether residual traces of the element remain in the brains of individuals who received contrast.
In the new study, Kanda and colleagues started with a pool of 190 subjects for whom autopsy was performed between 2010 and 2013 at the facility. Exclusion criteria whittled the cohort to five randomly selected patients who were given gadolinium contrast more than twice (the GBCA group). These subjects received gadopentetate dimeglumine (Magnevist, Bayer HealthCare); gadodiamide (Omniscan, GE Healthcare); or gadoteridol (ProHance, Bracco). They received a dose of 0.1 mmol/kg of body weight for each examination.
The study also included five people as a control group who had never received any contrast material (non-GBCA group). None of the subjects in either group had a history of severe renal dysfunction or acute renal failure.
The researchers used inductively coupled plasma mass spectroscopy (ICP-MS) to evaluate the accumulation of gadolinium in brain tissues, including the dentate nucleus and globus pallidus. Both the dentate nucleus and globus pallidus control the body's voluntary movements.
Upon analysis, the researchers found traces of gadolinium in specimens from all five people in the GBCA group (mean, 0.25 µg/g of brain tissue), with significantly higher concentrations in each region, compared with those who never had gadolinium contrast. In addition, in the GBCA group, the dentate nucleus and globus pallidus showed significantly higher gadolinium concentrations than other regions of the brain.
Most notably, the presence of gadolinium accumulation was discovered in subjects with no history of severe renal dysfunction.
"Because gadolinium is not an essential element in nature, its presence in the body is almost certainly due to GBCA administration," the authors wrote.
MRI signal intensity
This most recent study follows research by Kanda and colleagues published in January, in which they reviewed 127 patients who underwent brain MRI with gadopentetate dimeglumine or gadoteridol. Two radiologists visually evaluated the area from the dentate nucleus to the cerebellum on unenhanced T1-weighted MRI.
Hyperintensity was found in the dentate nucleus on unenhanced T1-weighted MR images, which the researchers associated with previous administration of gadopentetate dimeglumine. However, there was no hyperintensity in patients who had received gadoteridol.
Once again, the researchers were at a loss to explain the results.
"The mechanism by which GBCA administration causes high signal intensity in the dentate nucleus on T1-weighted MR image remains unclear," Kanda and colleagues wrote in that study. "Although gadolinium deposition occurs in the human brain independent of renal function, deposition may depend on the gadolinium chelate used" (Radiology, January 27, 2015).
Heidelberg study
German researchers found themselves in a similar quandary regarding the clinical implications of their GBCA study, published online April 6 in Radiology. Their results, however, at least confirmed findings in the January study by Kanda et al.
Lead author Dr. Alexander Radbruch and colleagues from University of Heidelberg Medical Center retrospectively compared signal intensity ratios of the dentate nucleus and the globus pallidus to ratios of other structures on unenhanced T1-weighted MRI.
The study included two groups of 50 patients who underwent at least six consecutive MRI scans with either gadopentetate dimeglumine or gadoterate meglumine (Dotarem, Guerbet).
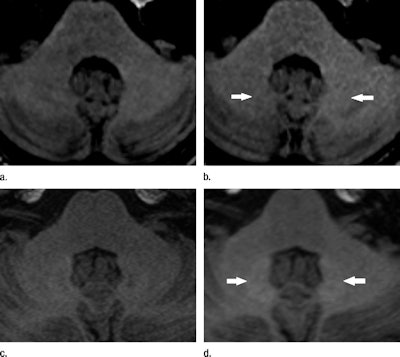 Unenhanced T1-weighted MRI shows hyperintensities in the dentate nucleus (arrows). Images were acquired before (a) and after (b) six administrations of gadopentetate dimeglumine and before (c) and after (d) six administrations of gadoterate meglumine. Images courtesy of Radiology.
Unenhanced T1-weighted MRI shows hyperintensities in the dentate nucleus (arrows). Images were acquired before (a) and after (b) six administrations of gadopentetate dimeglumine and before (c) and after (d) six administrations of gadoterate meglumine. Images courtesy of Radiology.The researchers found greater signal intensity in the dentate nucleus and globus pallidus on T1-weighted images among patients who received gadopentetate dimeglumine, while there was no such escalation in patients who had received gadoterate meglumine -- despite the fact that a substantially larger dose of contrast was used in the latter group.
And like Kanda's group, Radbruch and colleagues said they didn't know whether this increase in signal intensity has any connection to clinical issues.
Mayo Clinic study
Researchers from the Mayo Clinic in Rochester, MN, also were unable to explain the gadolinium traces they found in four areas of the brain several years after administration of MRI contrast (Radiology, March 5, 2015).
Their study recorded between 0.1 µg and 58.8 µg of gadolinium per gram of tissue in the four brain regions of the patients, who had relatively normal renal function at the time of their MRI exams.
Lead author Dr. Robert McDonald, PhD, and colleagues noted that deposits appeared to occur in all patients exposed to gadolinium and were detectable with as few as four doses of GBCA. But they also said that additional studies are needed to determine clinical significance.
Burden of proof
Kanal believes the burden of proof to demonstrate the safety of gadolinium contrast should be on the healthcare providers prescribing the agents.
"No one is going to take these agents away," he said. "We are so dependent upon them; they are so frighteningly powerful for society. I am not concerned about losing these agents, but I think it is appropriate to be concerned about whether we should be using all of these agents."