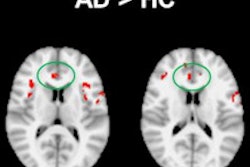
Interventional device developer Medtronic has received U.S. Food and Drug Administration (FDA) approval for use of its SureScan pacing systems with MRI scans positioned on any region of the body.
The SureScan systems are the first and only pacing systems in the U.S. with FDA clearance for use with MRI, Medtronic said.
Patients implanted with Medtronic's Advisa DR MRI or Revo MRI SureScan pacing systems can now have MRI scans without positioning restrictions, including the chest area, which previously had been restricted.
The recent approval comes after an FDA review of computer modeling and clinical data confirming that MRI chest-positioned scans are safe for patients.