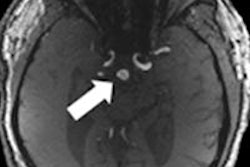
C4 Imaging has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Sirius MRI marker.
The marker will be used after treatment of prostate cancer with brachytherapy to facilitate seed localization within the prostate, utilizing a single postimplant MRI procedure.
The Sirius marker is visible with T1- and T2-weighted signals, according to the company.