Siemens Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its CAIPIRINHA software, which cuts by half the length of time patients need to hold their breath during MRI scans.
The CAIPIRINHA (controlled aliasing in parallel imaging results in higher acceleration) software is part of Siemens' syngo MR D13A software package for parallel MRI scanning. The protocol enables acquisition of higher-quality 3D volumetric interpolated breath-hold sequence (VIBE) T1 images through higher acceleration factors, according to the company.
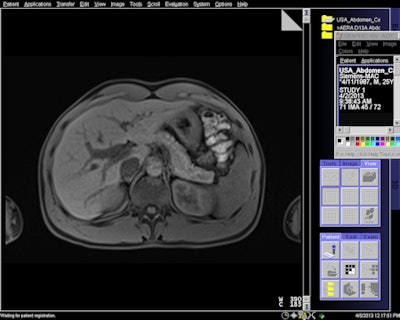
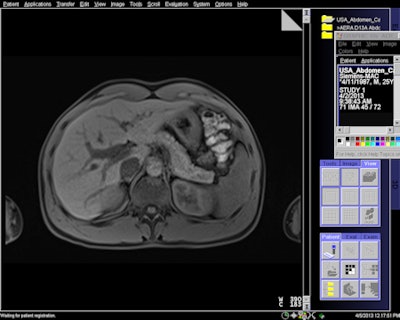 MR image acquired with 7-sec breath-hold with Siemens' CAIPIRINHA software.
MR image acquired with 7-sec breath-hold with Siemens' CAIPIRINHA software.
MR image acquired with 7-sec breath-hold with Siemens' CAIPIRINHA software.
CAIPIRINHA will be included as a standard feature with Siemens' D13A software for the Magnetom Avanto 1.5-tesla and Magnetom Verio 3-tesla scanners.