Contrast agent developer Bracco has received word that the U.S. Food and Drug Administration (FDA) has approved an expanded use for the company's MultiHance MR contrast agent.
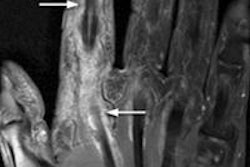
MultiHance is now approved for MR angiography (MRA) to evaluate adults with known or suspected renal or aortoiliofemoral occlusive vascular disease, Bracco said. The approval was granted based on data submitted to the FDA that included safety and efficacy results from two prospective, multicenter clinical trials.
Assessment of diagnostic efficacy for detecting and excluding clinically significant steno-occlusive disease (51% or greater stenosis) was based on comparisons of sensitivity and specificity between MultiHance MRA and noncontrast MRA, according to the company.
Results from both trials showed a statistically significant increase in both sensitivity and specificity of MultiHance MRA over noncontrast MRA in detecting clinically significant steno-occlusive disease, Bracco noted.