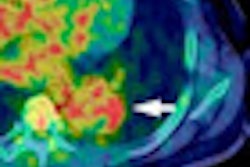
Radiopharmaceutical firm Ion Beam Applications (IBA) of Louvain-la-Neuve, Belgium, and Wilex of Munich, Germany, say a clinical study under way shows that PET/CT with the radiolabeled antibody Redectane can lead to significantly improved diagnosis of renal masses when compared to CT alone.
In a phase III study, a total of 226 patients suspected of having kidney cancer were enrolled in more than 14 centers in the U.S. Patients also were scheduled for complete or partial surgical removal of the affected kidney. They were imaged with CT alone and with PET/CT and Redectane prior to surgery to determine if they had clear cell renal cell carcinoma.
The correct diagnosis and presence of clear cell renal cell cancer was reached with statistical significance (p ≤ 0.016) compared to CT. The study also confirmed the absence of clear cell renal cell cancer with a high statistical significance (p < 0.001). Redectane also achieved sensitivity of 86% and specificity of 87%.
Based on the results, Wilex plans to submit the radiolabeled antibody to the U.S. Food and Drug Administration (FDA) for regulatory review at the end of this year. Additional details will be presented on June 1 at the American Urological Association (AUA) annual meeting in San Francisco.
Related Reading
IBA's sales decline in Q1, May 12, 2010
IBA warns of 2009 loss, February 22, 2010
IBA scores financing, December 18, 2009
IBA wins court order to reapply for contract, December 4, 2009
IBA lands Italian install, December 3, 2009
Copyright © 2010 AuntMinnie.com