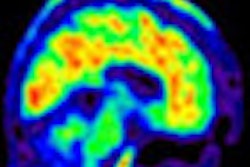
Europe's Committee for Medicinal Products for Human Use (CHMP) has recommended approval of Amyvid (florebetapir F-18) for injection as a PET radiotracer to detect beta-amyloid neuritic plaque in patients with cognitive impairment, according to Eli Lilly and its subsidiary Avid Radiopharmaceuticals.
CHMP's positive opinion will now be referred for final action to the European Commission, which has the authority to approve medicines for European Union markets, a process that usually takes three or fewer months, the companies said.
Amyvid should be used in conjunction with a clinical evaluation in patients being evaluated for Alzheimer's disease, according to the companies. It is intended as an adjunct to other diagnostic tests for adult patients being evaluated for Alzheimer's disease and other causes of cognitive impairment.