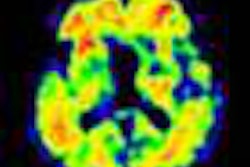
Radiopharmaceutical developer Navidea Biopharmaceuticals has enrolled the first patient in a phase II trial of its fluorine-18 NAV4694 radiopharmaceutical candidate, which is designed as an aid in detecting cerebral beta-amyloid plaque in subjects diagnosed with probable Alzheimer's disease (AD).
The study will compare images from subjects with probable AD with similarly aged and young healthy volunteers, according to Navidea. Two trial sites are currently enrolling subjects: Molecular NeuroImaging in New Haven, CT, and the Alzheimer's Disease Center at Quincy Medical Center in Quincy, MA.
In addition to safety and efficacy objective, the study will also measure the correlation between neuropsychiatric test results with the amount of beta amyloid determined by NAV4694 imaging, according to Navidea. The company plans to launch a phase III trial of NAV4694 in early 2013.